Kavakavaresin
- CAS NO.:9000-38-8
- Empirical Formula: C14H16O3
- Molecular Weight: 232.27504
- MDL number: MFCD02112945
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-18 17:43:10
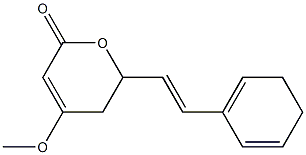
What is Kavakavaresin?
Description
Kava or Piper methysticum is a shrub of the pepper family,
native to Micronesia, Melanesia, and Polynesia. There are
approximately 150 different cultivars with different content
and composition of active ingredients, consequently resulting
in different intoxicating effects after ingestion. On
average, the kava lactones account for 3–20% of dry weight
of the kava root and have relatively low solubility in water.
The active components of kava are mostly contained in the
lipid-soluble resin where the lactones account for approximately
96%.
lactones varies according to the plant parts and the kava species
used for extraction. The aerial parts of the shrub contain
a relatively higher amount of alkaloids and are generally
avoided in traditional preparations.
Traditionally, extractions are made with cold water or
coconut milk from macerated dry or fresh root. Due to the low
water solubility of the lactones, commercial kava products
such as herbal supplements are made from organic solvents
(i.e., acetone and ethanol).
During the 1990s, kava became very popular in Western
countries resulting in increased import and demand. Following this popularity boom, a number of suspected kava-induced
hepatotoxic events were reported. Kava preparations were
banned in a number of European countries and the US Food
and Drug Administration (FDA) issued warnings.
The Uses of Kavakavaresin
Aqueous extracts from the root of kava has been used for
centuries in the South Pacific. The extract is used as a ceremonial
and intoxicating drink, but has also been used as a medicine for
various illnesses, including migraines and bladder disorders.
The pharmacologically active compounds are the kava lactones,
which allegedly possess analgesic, anticonvulsive, spasmolytic,
and antimycotic effects.
In Western countries, organic kava extracts have gained
popularity in the twentieth century as herbal supplements for
treating anxiety and insomnia.
Biological Activity
Kavakavaresin is a synthetic drug that inhibits the activity of the polymerase chain reaction.
Metabolism
Not Available
Toxicity evaluation
Mechanisms of action to explain kava’s pharmacological and
toxicological properties are still incompletely understood.
Known side effects are relatively mild and include allergic
reactions and gastrointestinal complaints. Kava dermopathy is
a well-recognized symptom in heavy kava users. It appears after
weeks of ingestion as a scaly rash; however, the skin returns to
normal upon cessation. There is an apparent dose–response
relationship and the prevalence in heavy kava users has been
reported to be 78%.
Amore serious issue is suspectedkava-induced hepatotoxicity.
This has resulted in extensive studies of kava lactones, chalcones,
and alkaloids. To date, there are no indisputable explanations
and, due to the lack of dose dependency, kava-induced hepatotoxicity
has been classified as idiosyncratic, possibly with
involvement of the immune system.
Ninety-three cases of suspected kava-induced hepatotoxicity
have been reviewed by the World Health Organization (WHO).
However, in most cases the information was inadequate to
determine whether hepatotoxicity was caused by kava. The
reported symptoms were hepatitis, hepatic failure, cholestatic
hepatitis, jaundice, abnormal hepatic function, and cirrhosis.
The outcome in seven of the reviewed cases was death and 14 of
the cases resulted in liver transplant. The mean duration to
onset was 111 days and positive rechallenge tests were seen in
five patients.
The form of kava use in the South Pacific and in Aboriginal
communities is apparently not associated with the hepatotoxicity
reported in Western countries. Elevated liver enzymes (gammaglutamyl
transferase (GGT) and alkaline phosphatase (ALP))
have been reported in these kava users, consuming an average
118 g week-1. The symptoms were reversible upon cessation.
Involvement of glutathione (GSH) and possibly depletion
of endogenous GSH has been proposed to be involved in the
toxic mechanism. A recent study revealed that in vitro toxicity
of kava and acetaminophen (APAP) was intensified by coadministration.
Toxicity of APAP is due to formation of a reactive
metabolite causing depletion of GSH. The increase in
toxicity when kava was coadministered suggests that GSH
somehow could be involved in detoxification of kava.
The possibility of kava acting as an inhibitor of specific
metabolic enzymes has been investigated. In vitro results have
shown that kava is indeed capable of altering the metabolic
capacity of a number of P-450 enzymes; however, the relation
to hepatotoxicity remains unclear.
Flavokavains have been identified as the most potent
cytotoxic compounds in kava. Recently, the flavokavains
gained attention as they have shown apoptotic effects on cancer
cells. Among others, the targets include nuclear factor kappa
beta (NF-kB), Bax, reactive oxygen species, and growth arrest
and DNA-damage-inducible protein (GADD153). Like the
lactones, these compounds have very low solubility in water.
Thus, an organic extract would be expected to contain much
higher concentrations than an aqueous extract. The toxicity of
flavokavains is yet to be examined in vivo.
Poor quality kava products have been accused of being
involved in the increased prevalence of kava-induced hepatotoxicity
in Western countries. Due to the popularity boom,
aerial parts of the shrub allegedly have been included in the
material used to produce commercial kava preparations.
Aerial parts are generally avoided in the South Pacific as they
contain a relatively high amount of alkaloids such as pipermethystine,
which has been reported to decrease cellular
ATP levels and mitochondrial membrane potential and
induce apoptosis in human HepG2 cells. Pipermethystine
has, however, not been identified in commercial products in
clinically significant amounts.
Another aspect of poor quality kava has recently been
addressed. It has been proposed that, due to high temperatures
and humidity in the South Pacific, mold would be able to
develop rapidly in kava plant material not stored correctly. It
has been suggested consequently that hepatotoxins including
aflatoxins could be present in poor quality kava plant material.
Properties of Kavakavaresin
| InChI | InChI=1S/C14H16O3/c1-16-13-9-12(17-14(15)10-13)8-7-11-5-3-2-4-6-11/h3,5-8,10,12H,2,4,9H2,1H3/b8-7+ |
| IARC | 2B (Vol. 108) 2016 |
Safety information for Kavakavaresin
Computed Descriptors for Kavakavaresin
| InChIKey | OMNGEVNATYFZGG-BQYQJAHWSA-N |
| SMILES | C1(/C=C/C2C=CCCC=2)OC(=O)C=C(OC)C1 |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1