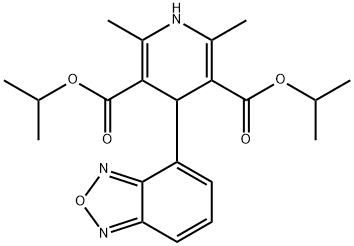
Isradipine
Synonym(s):4-(4-Benzofurazanyl)-1,-4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylic acid methyl 1-methylethyl ester;Isradipine - CAS 75695-93-1 - Calbiochem;L-type voltage-gated Ca2+ channel blocker, Isradipine, PN 200-110
- CAS NO.:75695-93-1
- Empirical Formula: C19H21N3O5
- Molecular Weight: 371.39
- MDL number: MFCD00153820
- EINECS: 630-420-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 11:34:44
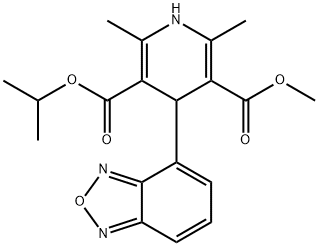
What is Isradipine?
Absorption
Isradipine is 90%-95% absorbed and is subject to extensive first-pass metabolism, resulting in a bioavailability of about 15%-24%.
Toxicity
Symptoms of overdose include lethargy, sinus tachycardia, and transient hypotension. Significant lethality was observed in mice given oral doses of over 200 mg/kg and rabbits given about 50 mg/kg of isradipine. Rats tolerated doses of over 2000 mg/kg without effects on survival.
Description
Isradipine is a long-acting calcium blocker effective in the treatment of essential hypertension. The advantages of isradipine as compared with other dihydropyridine calcium antagonists include a "gentle" onset and fewer contraindications. It can be taken by patients with diabetes mellitus, chronic obstructive airway diseases, gout, congestive heart failure or ischemic heart disease.
Chemical properties
Yellow Solid
Originator
Sandoz (Switzerland)
The Uses of Isradipine
Isradipine is a L-type calcium channel blocker, Isradipine has anti-atherosclerotic effects in animals and improves endothelium-mediated nitric oxide (NO)-dependent vasodilation in vitro. Additional studies indicate that Isradipine may provide a neuroprotective effect in a rat model of focal ischemia and that Isradipine produces a dose-dependent sparing of dopamine neuronal fibers and cell bodies.
The Uses of Isradipine
Dihydropyridine calcium channel blocker. Antihypertensive; antianginal
The Uses of Isradipine
nonsedating H1-antihistamine
Background
Isradipine belongs to the dihydropyridine (DHP) class of calcium channel blockers (CCBs), the most widely used class of CCBs. It is structurally related to felodipine, nifedipine, and nimodipine and is the most potent calcium-channel blocking agent of the DHP class. Isradipine binds to calcium channels with high affinity and specificity and inhibits calcium flux into cardiac and arterial smooth muscle cells. It exhibits greater selectivity towards arterial smooth muscle cells owing to alternative splicing of the alpha-1 subunit of the channel and increased prevalence of inactive channels in smooth muscle cells. Isradipine may be used to treat mild to moderate essential hypertension.
Indications
For the management of mild to moderate essential hypertension. It may be used alone or concurrently with thiazide-type diuretics.
What are the applications of Application
Isradipine is a selective agonist and calcium channel protein inhibitor
Definition
ChEBI: Isradipine is an isopropyl ester, a methyl ester, a dihydropyridine and a benzoxadiazole.
brand name
Dynacirc (Reliant);Prescal.
General Description
Isradipine, 4-(4-benzofuranazyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinecarboxylic acid methyl 1-methylethyl ester (DynaCirc), is another second-generationdihydropyridine-type channel blocker. This drug, like theother second-generation analogs, is more selective for vascularsmooth muscle than for myocardial tissue. It is effectivein the treatment of stable angina, reducing the frequencyof anginal attacks and the need to use nitroglycerin.
Biological Activity
Potent and selective L-type voltage-gated Ca 2+ channel blocker. EC 50 and pA 2 values are 1.4 nM and 10.3 for relaxation of depolarization- and Ca 2+ -induced contractions of rabbit aorta respectively; EC 25 = 0.45 nM for reduction in rate of spontaneously beating guinea pig right atria. Long-acting antihypertensive agent in vivo , exerting primary effects on vascular tissue with secondary negative chronotropic action.
Biochem/physiol Actions
L-type calcium channel blocker (also referred to as dihydropyridine-type calcium channel blocker); antihypertensive.
Pharmacokinetics
Isradipine decreases arterial smooth muscle contractility and subsequent vasoconstriction by inhibiting the influx of calcium ions through L-type calcium channels. Calcium ions entering the cell through these channels bind to calmodulin. Calcium-bound calmodulin then binds to and activates myosin light chain kinase (MLCK). Activated MLCK catalyzes the phosphorylation of the regulatory light chain subunit of myosin, a key step in muscle contraction. Signal amplification is achieved by calcium-induced calcium release from the sarcoplasmic reticulum through ryanodine receptors. Inhibition of the initial influx of calcium decreases the contractile activity of arterial smooth muscle cells and results in vasodilation. The vasodilatory effects of isradipine result in an overall decrease in blood pressure.
Metabolism
Hepatic. Completely metabolized prior to excretion and no unchanged drug is detected in the urine.
Storage
Room temperature
References
1) Bracht et al. (2001), Isradipine improves endothelium-dependent vasodilation in normotensive coronary artery disease patients with hypercholesterolemia; J. Hyperten., 19 899 2) Chandra et al. (1999), Use of diffusion-weighted MRI and neurological deficit scores to demonstrate beneficial effects of isradipine in a rat model of focal ischemia ; Pharmacology, 58 292 3) Merck 14:5240
Properties of Isradipine
| Melting point: | 166-1680C |
| Boiling point: | 501.9±60.0 °C(Predicted) |
| Density | 1.249±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: ~26 mg/mL |
| form | solid |
| pka | 2.56±0.70(Predicted) |
| color | yellow |
| Merck | 14,5240 |
| Stability: | Stable for 2 years from date of purchsae as supplied. Protect from moisture. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 75695-93-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Isradipine(75695-93-1) |
Safety information for Isradipine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
Computed Descriptors for Isradipine
| InChIKey | HMJIYCCIJYRONP-UHFFFAOYSA-N |
Isradipine manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 75695-93-1 Isradipine 98%View Details
75695-93-1 Isradipine 98%View Details
75695-93-1 -
 75695-93-1 98%View Details
75695-93-1 98%View Details
75695-93-1 -
 Isradipine 99%View Details
Isradipine 99%View Details
75695-93-1 -
 Isradipine CAS 75695-93-1View Details
Isradipine CAS 75695-93-1View Details
75695-93-1 -
 Isradipine 98% (HPLC) CAS 75695-93-1View Details
Isradipine 98% (HPLC) CAS 75695-93-1View Details
75695-93-1 -
 Isradipine CAS 75695-93-1View Details
Isradipine CAS 75695-93-1View Details
75695-93-1 -
 Isradipine CAS 75695-93-1View Details
Isradipine CAS 75695-93-1View Details
75695-93-1 -
 Isradipine CAS 75695-93-1View Details
Isradipine CAS 75695-93-1View Details
75695-93-1
