ISOXANTHOPTERIN
Synonym(s):2-Amino-4,7-dihydroxy-1,3,5,8-tetraazanaphthalene;2-Amino-4,7-dihydroxypteridine;2-Amino-4,7-pteridinediol
- CAS NO.:529-69-1
- Empirical Formula: C6H5N5O2
- Molecular Weight: 179.14
- MDL number: MFCD00006696
- EINECS: 208-469-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-25 18:01:24
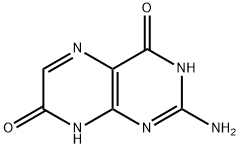
What is ISOXANTHOPTERIN?
Chemical properties
Off-white powder
The Uses of ISOXANTHOPTERIN
Isoxanthopterin is a substrate for the enzyme isoxanthopterin deaminase.
What are the applications of Application
Isoxanthopterin is an oxidation product of pterin via xanthine oxidase
Definition
ChEBI: 2-aminopteridine-4,7-diol is a dihydroxypteridine.
Synthesis Reference(s)
Journal of the American Chemical Society, 75, p. 4450, 1953 DOI: 10.1021/ja01114a016
Biological Activity
isoxanthopterin interferes with rna and dna synthesis.isoxanthopterin, a natural intermediate in the pteridine pathway, plays critical roles in pigmentation and cell division.
in vitro
isoxanthopterin was identified as a pteridine of wide natural occurrence and was reported to exerts an inhibitory action on the synthesis of ribosomal rna, possibly soluble rna and on dna in developing eggs of the milkweed bug oncopeltus fasciatus. such effects were measured by partially incubating dechorionated eggs in a solution containing isoxanthopterin and radioactive uridine or thymidine, and then testing the incorporation of the isotope into ribosomal rna or dna. the detailed mechanism of this inhibition had been discussed, and the tentative conclusion was agreed that it was due to an interaction between the pteridine and the nucleic acid (most probably dna) [1].
in vivo
previous animal study showed that rats with inherited retinal dystrophy appeared to differ from healthy rats by an elevated isoxanthopterin excretion in urine. rcs rats responded to feeding of monapterin with an increase of isoxanthopterin excretion. this phenomenon supported the idea that the rcs rat had an increased xanthinoxidase activity compared with healthy rats [2].
Purification Methods
Purify it by repeated precipitation from alkaline solution with acid (preferably AcOH or formic acid), filter, wash well with H2O, then EtOH and dry at 100o. The purity is checked by paper chromatography [RF 0.15 (n-BuOH/AcOH/H2O, 4:1:1); 0.33 (3% aqueous NH4OH). [Goto et al. Arch Biochem Biophys 111 8 1965.] [For biochemistry see Blakley Biochemistry of Folic Acid and Related Pteridines North Holland Publ Co, Amsterdam 1969.] [Beilstein 26 III/IV 3999.]
References
[1] lagowski, j. m. and forrest, h.s. interaction in vitro between isoxanthopterin and dna. proceedings of the national academy of sciences of the united states of america 58(4), 1541-1547 (1967).
[2] g. cremer-bartels, h. gerding, l. hanneken and k. krause. isoxanthopterin excretion of rats with inherited retinal dystrophy. pteridines vol. 2, 1990, pp. 103 -105.
Properties of ISOXANTHOPTERIN
| Melting point: | >300 °C |
| Density | 2.17±0.1 g/cm3(Predicted) |
| storage temp. | room temp |
| solubility | DMSO (Very Slightly) |
| form | powder |
| pka | 11.32±0.20(Predicted) |
| color | off-white |
| BRN | 181594 |
Safety information for ISOXANTHOPTERIN
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P362:Take off contaminated clothing and wash before reuse. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for ISOXANTHOPTERIN
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran






You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8