Iron Chelator, Dp44mT
Synonym(s):2-(Di-2-pyridinylmethylene)-N,N-dimethyl-hydrazinecarbothioamide;Di-2-pyridylketone-4,4,-dimethyl-3-thiosemicarbazone
- CAS NO.:152095-12-0
- Empirical Formula: C14H15N5S
- Molecular Weight: 285.37
- MDL number: MFCD20527329
- EINECS: 694-427-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-08 09:02:48
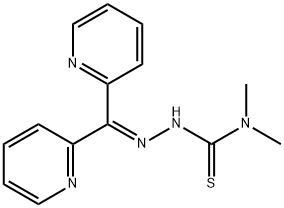
What is Iron Chelator, Dp44mT?
Description
Dp44mT (152095-12-0) is a metal chelator with potent antitumor activity.1,2?It displayed an average IC50?of 30 nM over 28 cancer cell lines (IC50?range was 5 nM to 400 nM).2?Dp44mT retained its antiproliferative activity in both etoposide-resistant MCF-7/VP clones (MCF-7 breast cancer cells) and vinblastine-resistant KB-VB1 clones (KB3-1 epidermoid carcinoma cells) with an IC50?= 12 nM for both lines.2?The potency of Dp44mT has been attributed to the high redox activity of the Dp44mT-Fe complex leading to cytotoxic ROS generation. The antitumor activity of Dp44mT may also be mediated by a redox active copper complex that causes cellular glutathione depletion and lysosomal damage.3,4?It also inhibited T-cell activation and prevented CD25 up-regulation?via?a copper-dependent mechanism.5?Dp44mT has recently been shown to effectively inhibit c-Met though metalloprotease-mediated cleavage and lysosomal degradation.6
The Uses of Iron Chelator, Dp44mT
Iron Chelator, Dp44mT is a cell-permeable di-2-pyridyl thiosemicarbazone (DpT) based tridentate ligand.
What are the applications of Application
Iron Chelator, Dp44mT is a cell-permeable di-2-pyridyl thiosemicarbazone (DpT) based tridentate ligand
Biochem/physiol Actions
Dp44mT (di-2-pyridylketone-4,4,-dimethyl-3-thiosemicarbazone) influences lysosome integrity through copper binding. It induces reactive oxygen species (ROS) generation by redox cycling of iron complex. Dp44mT exhibits anti cancer action by attenuating Ndrg-1 (N-myc downstream regulated 1), a metastasis suppressor protein. It also alters the cyclin family of proteins (A, B, D1, D2,D3 and cyclin-dependent kinase 2) known for cell-cycle regulation. Dp44mT is known to promote apoptosis in neuroepithelioma, melanoma and breast cancer.
in vivo
6 and 24 hours after the treatment with 0.1 and 1 μmol/l of dp44mt, the treatment resulted in the covalent complex formation between dna and top2α. no complex formation was found after the treatment when probed for top1 or top2β. caspase inhibitor pretreatment did not rescue the formation of top2α complex, so the formed top2α-dna complexes were not the secondary effect of apoptosis [1].
References
1) Yuan?et al.?(2004),?Novel di-2-pyridyl-derived iron chelators with marked and selective antitumor activity: in vitro and in vivo assessment;?Blood,?104?1450 2) Whitnall?et al.?(2006),?A class of iron chelators with a wide spectrum of potent antitumor activity that overcomes resistance to chemotherapeutics., Proc. Natl. Acad. Sci. USA?103?14910 3) Lovejoy?et al. (2011),?Antitumor activity of metal-chelating compound Dp44mT is mediated by formation of a redox-active copper complex that accumulates in lysosomes; Cancer Res.,?71?5871 4) Gutierrez?et al.?(2014),?The anticancer agent di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbzone (Dp44mT) overcomes prosurvival autophagy by two mechanisms: persistent reduction of autophagosome synthesis and impairment of lysosomal integrity; J. Biol. Chem.,?289?33568 5) Gundelach?et al.?(2013),?The anticancer drug Dp344mT inhibits T-cell activation and CD25 through a copper-dependent mechanism; FASEB J.,?27?782 6)Park?et al. (2020),?Thiosemicarbazones suppress expression of the c-Met oncogene by mechanisms involving lysosomal degradation and intracellular shedding;?J. Biol.Chem.,?295?481
Properties of Iron Chelator, Dp44mT
| storage temp. | 2-8°C |
| solubility | DMSO: ≥5mg/mL |
| form | powder |
| color | yellow to orange |
| Sensitive | Light Sensitive |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may ne stored at -20°C for 1 month. |
Safety information for Iron Chelator, Dp44mT
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Iron Chelator, Dp44mT
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Dp44mT CAS 152095-12-0View Details
Dp44mT CAS 152095-12-0View Details
152095-12-0 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1