Irisone
- CAS NO.:14901-07-6
- Empirical Formula: C13H20O
- Molecular Weight: 192.3
- MDL number: MFCD00001549
- EINECS: 238-969-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
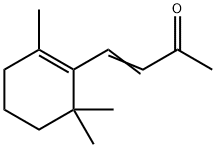
What is Irisone?
Description
β-Ionone has a characteristic violet-like odor, more fruity and woody than α-ionone. May be prepared by condensing citral with acetone to form pseudoionone, which is then cyclized by acid-type reagents.
Description
Why do fresh home-grown tomatoes taste so much better than store-bought ones? The answer lies in a genetic modification that was introduced into commercial tomatoes to give them a longer shelf life. The downside of this mutation is that it reduces flavor and sugar development in the fruit.
Researchers in several nations led by Harry Klee at the University of Florida (Gainesville) and Sanwen Huang at the Chinese Academy of Agricultural Sciences (Beijing) determined how to modify mass-produced tomatoes?to restore desirable flavor components. The research team’s goal was to tell breeders which biochemicals are lacking in their tomatoes and to show them the markers they need to breed back in.
Based on the results of a human taste panel study, they identified 13 flavor molecules that are deficient in supermarket tomatoes. Two of the desired flavoring agents, geranylacetone?(upper images) and β-ionone (lower images), contribute “leafy” and “floral” flavors, respectively. The researchers then identified the regions of the tomato genome that are responsible for producing the 13 components.
Commenting on this study, James Giovannoni at the US Department of Agriculture said, “I don’t know if it’s possible to make a supermarket tomato that tastes exactly like it was grown in your own garden, but I have no doubt that this work can help breeders make supermarket tomatoes a lot better than they are now.”
Chemical properties
clear slightly yellow to yellow liquid
Chemical properties
β-Ionone has a characteristic violet-like odor, more fruity and woody than α-ionone
Occurrence
Reported found in the distillate from flowers of Boronia megatisma Nees. Also reported found in apricot, orange juice, lemon peel oil, guava, grapes, melon, papaya, peach, raspberry, blackberry, carrot, peas, bell pepper, tomato, ginger, peppermint and spearmint oil, milk powder, beef, hop oil, cognac, whiskies, grape wines, tea, passion fruit, plum, beans, mushroom, starfruit, almonds, mango, fenugreek, tamarind, apple and pear brandy, rice bran, quince, prickly pear, sweet potato, buckwheat, corn oil, corn tortillas, loquat, mountain papaya, clary sage and other sources
The Uses of Irisone
beta-Lonone is an essential oil extract from the leaves and flowers of Succisa Pratensis that shows antioxidant and antimicrobial activities against bacteria.
The Uses of Irisone
β-Ionone may be used as a reference standard in the determination of β-ionone in wine using solid-phase extraction followed by gas chromatography with mass spectrometry (GC-MS).
Preparation
By condensing citral with acetone to form pseudoionone, which is then cyclized by acid-type reagents.
Definition
ChEBI: Beta-ionone is an ionone that is but-3-en-2-one substituted by a 2,6,6-trimethylcyclohex-1-en-1-yl group at position 4. It has a role as an antioxidant and a fragrance.
Aroma threshold values
Detection: 0.007 to 205 ppb
Taste threshold values
Taste characteristics art 10 ppm: woody, sweet, fruity, berry-like with a green berry background
General Description
Colorless to light yellow liquid with an odor of cedar wood. In very dilute alcoholic solution the odor resembles odor of violets. Used in perfumery.
Air & Water Reactions
Slightly water soluble .
Reactivity Profile
Irisone may react vigorously with oxidizing agents. May react exothermically with reducing agents to release hydrogen gas.
Health Hazard
ACUTE/CHRONIC HAZARDS: Toxic. May cause allergic reaction.
Flammability and Explosibility
Non flammable
Biochem/physiol Actions
Taste at 1-20 ppm
Properties of Irisone
| Melting point: | -49°C |
| Boiling point: | 126-128 °C12 mm Hg(lit.) |
| Density | 0.945 g/mL at 25 °C(lit.) |
| vapor pressure | 1.706Pa at 25℃ |
| refractive index | n |
| FEMA | 2595 | BETA-IONONE |
| Flash point: | 230 °F |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly) |
| form | Liquid |
| pka | 0[at 20 ℃] |
| color | Clear slightly yellow to yellow |
| Odor | at 10.00 % in dipropylene glycol. floral woody sweet fruity berry tropical beeswax |
| Water Solubility | SLIGHTLY SOLUBLE |
| JECFA Number | 389 |
| Merck | 14,5056 |
| BRN | 1909544 |
| Dielectric constant | 12.0(20℃) |
| Stability: | Light Sensitive |
| CAS DataBase Reference | 14901-07-6(CAS DataBase Reference) |
| NIST Chemistry Reference | 4-(2,6,6-Trimethylcyclohex-1-en-1-yl)but-3-en-2-one(14901-07-6) |
| EPA Substance Registry System | 4-(2,6,6-Trimethyl-1-cyclohexenyl)-3-buten-2-one (14901-07-6) |
Safety information for Irisone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Irisone
Irisone manufacturer
Moraya GlobaL Private Limited
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

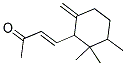

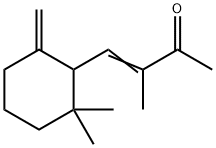
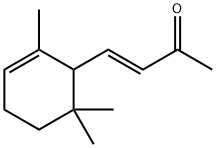
You may like
-
 14901-07-6 BETA IONONE 98%View Details
14901-07-6 BETA IONONE 98%View Details
14901-07-6 -
 14901-07-6 98%View Details
14901-07-6 98%View Details
14901-07-6 -
 beta-Ionone 98%View Details
beta-Ionone 98%View Details
14901-07-6 -
 β-Ionone CAS 14901-07-6View Details
β-Ionone CAS 14901-07-6View Details
14901-07-6 -
 β-Ionone CAS 14901-07-6View Details
β-Ionone CAS 14901-07-6View Details
14901-07-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1