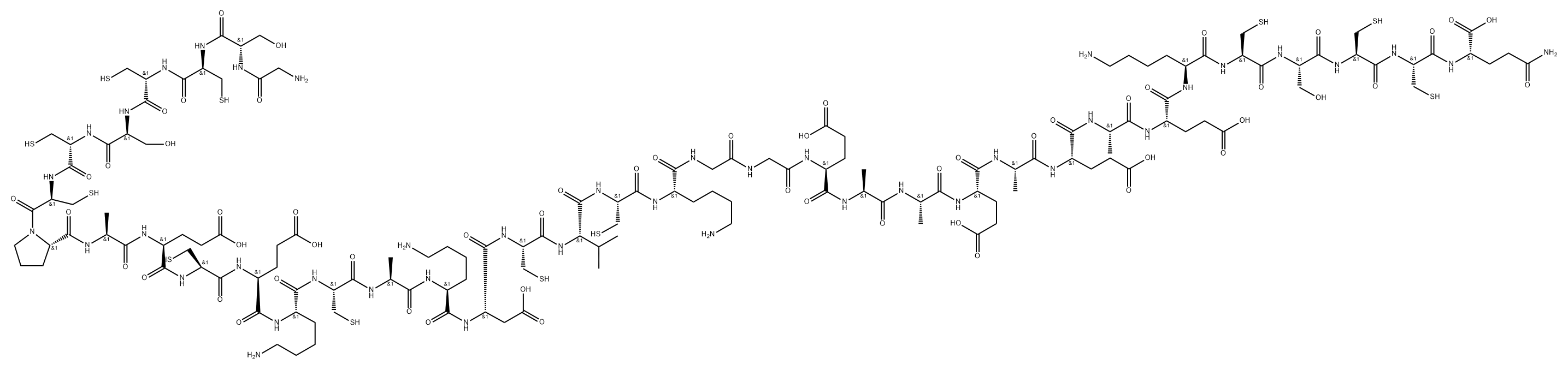
INSULIN
Synonym(s):Bovine insulin;Insulin human;Insulin, Zinc, Human, Recombinant, P. pastoris;Cell culture grade insulin;Insulin from hog pancreas
- CAS NO.:11061-68-0
- Empirical Formula: C257H383N65O77S6
- Molecular Weight: 0
- MDL number: MFCD00131380
- EINECS: 234-279-7
- Update Date: 2024-11-19 15:53:33
What is INSULIN?
Description
Insulin (INS) is the only hormone lowering blood glucose levels in vertebrates. However, the importance of insulin in the carbohydrate metabolism may differ between mammals and other vertebrates. Frederick Banting and Charles Best discovered a substance that lowered blood sugar levels in the dog pancreas in 1921, and this was immediately applied to diabetes care. The primary structure of INS has been reported in many vertebrates, including agnathans, fish, and tetrapods.
Chemical properties
White or almost white powder.
The Uses of INSULIN
Insulin shows a broad range of activities on a variety of somatic cells. Recombinant human insulin can be used to stimulate growth and proliferation of cultured cells and to investigate insulin activity on sensitive cells used in research studies. It is also a component of serum-free media formulations for most primary cells and cell lines.
The Uses of INSULIN
Antidiabetic.
What are the applications of Application
Insulin is a molecule produced in E.coli
What are the applications of Application
Human Insulin, Solution is derived from Escherichia Coli., 10mg/ml, 0.2μm filtered, for use in cell culture applications
brand name
Humulin (Lilly); Novolin (Novo Nordisk); Velosulin (Novo Nordisk).
General Description
The INS gene encodes for preproinsulin, which is enzymatically converted into insulin. Insulin is produced in the insulin-producing pancreatic β cells. Preproinsulin is converted to proinsulin in ER and proinsulin is then proteolytically processed to form insulin in newly-forming insulin secretory granules. Insulin production is tightly regulated by specific DNA elements present within ~400 bp in the proximal region of the INS promoter.
Biochem/physiol Actions
Insulin is responsible for two types of actions- excitatory and inhibitory. In its excitatory role, it increases the uptake of glucose and lipid synthesis, and in its inhibitory role it inhibits glycogenolysis, gluconeogenesis, lipolysis, proteolysis and ketogenesis. Aberrant insulin secretion leads to various disorders such as diabetes, hyperglycemia or hypoglycaemia. Type I diabetes is a result of autoimmune destruction of β cells of pancreas, which leads to depletion of insulin. Mutant INS-gene Induced Diabetes of Youth (MIDY) syndrome is an autosomal dominant disorder caused by missense mutations, which lead to aberrant proinsulin folding. Impaired glucose tolerance (IGT) or non-insulin-dependent diabetes mellitus (NIDDM) is caused by resistance to insulin-stimulated glucose uptake.
Clinical Use
In mammals and chickens, INS levels are routinely measured using commercial kits, and several studies have established specific immunoassays for fish. Many INS analogs, which show different durations of action, have been developed to treat diabetes. Alternative INS administration methods other than injection are via the lungs, nose, skin, and mouth. These have been developed using the insulin encapsulation technique with micro- or nanodelivery systems. Many medical drugs that stimulate INS release, both directly and indirectly, are commercially available. Sulfonylurea and glinides stimulate β-cells directly. The incretin GLP-1 analog can lower glucose concentrations by augmenting insulin secretion and suppressing glucagon release. In addition the dipeptidyl peptidase-4 (DPP-4) inhibitor inhibits incretin degradation and indirectly enhances the stimulation of incretin to β-cells. Thiazolidinedione improves the action of INS in the muscle and adipose tissue. Cone snail (Conus geographus) G1 is a naturally occurring B-chainminimized mimetic of INS, which strongly binds to human and fish INSR and activates receptor signaling. This peptide induces extremely rapid hypoglycemic shock.
in vitro
methotrexate (mtx) was found to be linked to insulin covalently. as effectively as insulin, insulin-mtx complex competed with 125i-insulin for insulin receptor. it was found that ic50 and ki for insulin-mtx were 93.82 nm and 91.88 nm, respectively, whereas those for insulin were 5.01 nm and 4.85 nm, respectively [1].
in vivo
previous study showed that insulin-stimulated glucose uptake in extensor muscles from sjl mice was reduced, but the basal uptake rates were not different. in another mouse study, knockdown of tc10α but not tc10β in 3t3-l1 adipocytes resulted in a inhibition of both insulin-stimulated glucose uptake and glut4 translocation [2].
storage
Store at -20°C
Structure and conformation
Human preproINS consists of 110 aa residues: 24 in the
signal peptide, 30 in the B-chain, 31 in the C-peptide, and
21 in the A-chain. The signal peptide and
C-peptide are excised from the preproINS to produce
the mature peptide, consisting of heterodimeric A- and
B-chains. Six cysteine residues are conserved throughout evolution from agnathans to mammals. Furthermore, the N-terminal seven aa residues of the A-chain
and the receptor binding region of the B-chain (Gly-Phe-Phe-Tyr) are highly conserved. Mr 5808, pI 5.3. INS is soluble under acidic and alkaline
conditions and almost insoluble at neutral pH. INS can be
stored for up to 6 months at 4°C in 1M acetic acid. Long-term
storage at an alkaline pH increases the rate of deamidation
and aggregation. One IU is equivalent to 0.0347mg
human INS.
References
[1] ou x,kuang a,liang z,peng x,zhong y. the binding characteristics of insulin-mtx to insulin receptor. hua xi yi ke da xue xue bao.2001 dec;32(4):538-40.
[2] leney se,tavaré jm. the molecular basis of insulin-stimulated glucose uptake: signalling, trafficking and potential drug targets. j endocrinol.2009 oct;203(1):1-18.
Properties of INSULIN
| Melting point: | >200°C (dec.) |
| storage temp. | -20°C |
| solubility | acidified water, pH 2.0: 2 mg/mL |
| form | solution |
| color | White |
| Water Solubility | Soluble up to 10mg/ml in pH <3 or in the presence of surfactants. Adjusting pH with a volatile acid (such as formic acid) prior to dry down will allow the product to be re-dissolved in water. |
Safety information for INSULIN
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for INSULIN
INSULIN manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 r-Insulin ex. Human for cell culture CAS 11061-68-0View Details
r-Insulin ex. Human for cell culture CAS 11061-68-0View Details
11061-68-0 -
 Insulin human CAS 11061-68-0View Details
Insulin human CAS 11061-68-0View Details
11061-68-0 -
 Insulin, human CAS 11061-68-0View Details
Insulin, human CAS 11061-68-0View Details
11061-68-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1