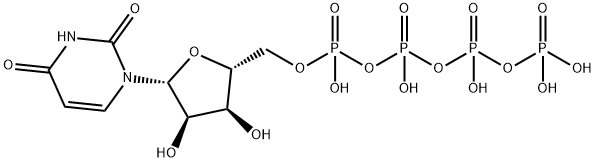
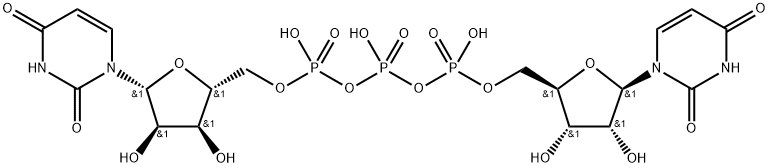
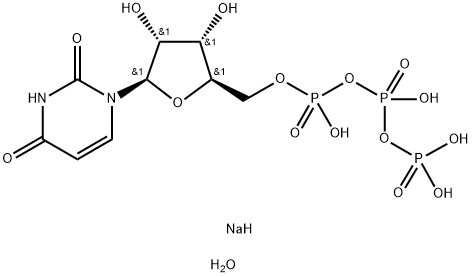
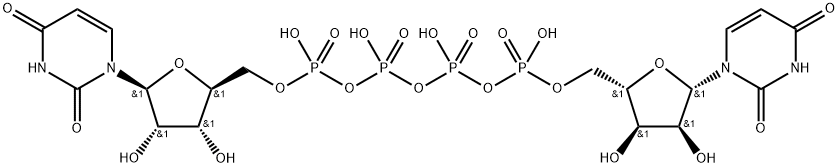
Diquafosol Tetrasodium
- CAS NO.:211427-08-6
- Empirical Formula: C18H27N4NaO23P4
- Molecular Weight: 814.3
- MDL number: MFCD18071356
- EINECS: 253-874-2
- Update Date: 2025-02-12 13:10:04
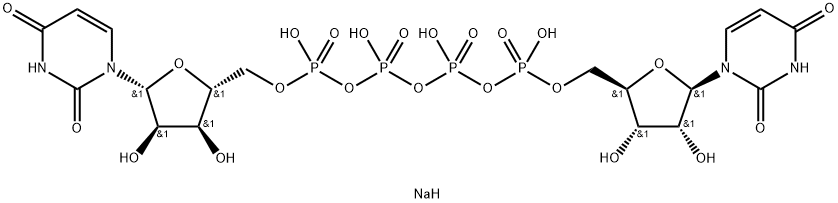
What is Diquafosol Tetrasodium?
Description
Diquafosol (INS-365) was approved in Japan in 2010 as a 3% ophthalmic solution for treatment of dry eye disease. Clinical diagnosis of dry eye is difficult because the condition presents a variety of symptoms. Treatment options include tear supplements (lubricants), anti-inflammatory drugs (e.g., cyclosporine eye drops or steroid eye drops), and tear retention devices. Diquafosol is a unique agent for the treatment of dry eye in that it acts as a P2Y2 purinergic receptor agonist with the ability to activate this receptor on the ocular surface and stimulate water, lipid, and mucin secretion. Diquafosol is metabolized by phosphodiesterases to UTP, UDP, UMP, and uridine. Diquafosol has been well tolerated in clinical trials, with side effects being local to the ocular surface.In addition,no serious ocular or systemic adverse drug reactions were found during the clinical trials.
Originator
Inspire Pharmaceuticals (United States)
The Uses of Diquafosol Tetrasodium
Enhancement of mucosal hydration in the treatment of chronic dry eye (P2Y2 receptor antagonist).
brand name
Diquas
Clinical Use
Diquafosol tetrasodium was approved in April 2010 as Diquas ® ophthalmic solution 3% for the treatment of dry eye syndrome and launched in Japan by Santen Pharmaceuticals. Diquafosol tetrasodium was originally discovered by Inspire Pharmaceuticals. In 2001, it was licensed to Santen for co-development and commercialization in Asian countries, and co-developed in collaboration with Allergan for the countries outside of Asia. In the U.S., diquafosol tetrasodium was submitted for a New Drug Application (NDA) as Prolacria ®(2% ophthalmic formulation) in June 2003. However, it is still in Phase III clinical development for dry eye syndrome. Diquafosol tetrasodium, also known as INS- 365, is a P2Y2 receptor agonist, which activates P2Y2 receptor on the ocular surface, leading to rehydration through activation of the fluid pump mechanism of the accessory lacrimal glands on the conjunctival surface.
Synthesis
The large-scale synthesis route of diquafosol tetrasodium is described in Scheme 4. Commercially available uridine 5?ˉ-diphosphate disodium salt (21) was transformed into the corresponding tributylamine salt by ion exchange chromatography on Dowex 50 using Bu3NH+ phase, and then dimerized by means of CDI in DMF at 50 oC. The crude product was purified by Sephadex DEAE column followed by ion exchange using a Dowex 50W resin in Na+ mode. The onepot process provided diquafosol tetrasodium (IV) in 25% yield.
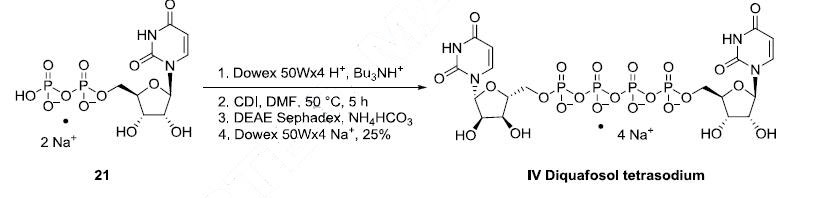
Properties of Diquafosol Tetrasodium
| storage temp. | Store at -20°C |
| solubility | DMSO:1.0(Max Conc. mg/mL);1.14(Max Conc. mM) Water:100.0(Max Conc. mg/mL);113.86(Max Conc. mM) |
| form | A solid |
| color | White to off-white |
Safety information for Diquafosol Tetrasodium
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H228:Flammable solids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P370+P378:In case of fire: Use … for extinction. |
Computed Descriptors for Diquafosol Tetrasodium
New Products
(R)-1-Boc-3-hydroxypyrrolidine Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran

You may like
-
 Diquafosol tetrasodium CAS 211427-08-6View Details
Diquafosol tetrasodium CAS 211427-08-6View Details
211427-08-6 -
![Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
61397-56-6 -
 287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
