Indinavir
Synonym(s):Indinavir monohydrate
- CAS NO.:150378-17-9
- Empirical Formula: C36H47N5O4
- Molecular Weight: 613.79
- MDL number: MFCD00866938
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-08-15 19:00:21
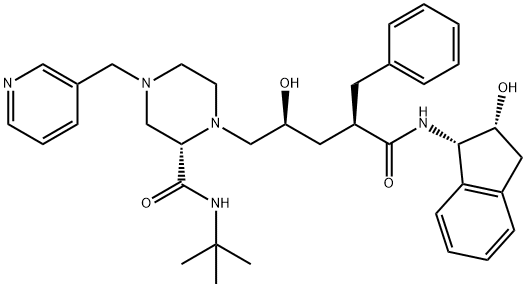
What is Indinavir?
Absorption
Rapidly absorbed
Toxicity
Symptoms of overdose include myocardial infarction and angina pectoris.
Description
Crixivan was launched in Australia, Switzerland, the UK and the US as an orally-bioavailable HIV-1 protease inhibitor. The compound can be prepared by coupling an optically active piperazine derivative with an epoxide derivative (now commercially available). The synthesis of the proteins, reverse transcriptase, integrase, structural proteins and a protease, required by the virus to complete its lifecycle, can be interupted if the protease enzyme is not capable of cleaving a proform polypeptide chain into these components. lndinavir inhibits this process and is more potent than the first approved protease inhibitor saquinavir. This effect was noted by the increase in CD4+ cells and a decrease in HIV RNA levels. Since indinavir is metabolized by the CYP3A4 isozyme, care must be taken with patients with hepatic insufficiency and to sex-related differences in the level of this enzyme. Other than nephrolithiasis (5%), indinavir is relatively safe and well tolerated.
Originator
Merck (USA)
The Uses of Indinavir
Indinavir is a member of the novel hydroxyaminopentane amide class of HIV-1 protease inhibitors. Indinavir is used as an antiviral.
The Uses of Indinavir
Indinavir is a member of the novel hydroxyaminopentane amide class of HIV-1 protease inhibitors. Indinavir is used as an antiviral. It is a COVID19-related research product.
Background
A potent and specific HIV protease inhibitor that appears to have good oral bioavailability. [PubChem]
Indications
Indinavir is an antiretroviral drug for the treatment of HIV infection.
What are the applications of Application
Indinavir is a member of the novel hydroxyaminopentane amide class of HIV-1 protease inhibitors
Indications
Indinavir (Crixivan) is a potent inhibitor of HIV reverse transcriptase. It produces the side effects common to all protease inhibitors and also may produce nephrolithiasis, urolithiasis, and possibly renal insufficiency or renal failure. This problem occurs more frequently in children (approximately 30%) than adults (approximately 10%) and can be minimized by drinking at least 1.5 L of water daily. Additional side effects include asymptomatic hyperbilirubinemia, alopecia, ingrown toenails, and paronychia. Hemolytic anemia rarely occurs. Rifampin should not be given with indinavir.
Definition
ChEBI: Indinavir is a N-(2-hydroxyethyl)piperazine, a piperazinecarboxamide and a dicarboxylic acid diamide. It has a role as a HIV protease inhibitor.
brand name
Crixivan
Acquired resistance
The major mutations in the protease enzyme associated with loss of the antiretroviral activity occur at positions 46, 82 and 84. Generally, the level of resistance rises with the number of point mutations.
General Description
When administered with a high-fat diet, indinavir(Crixivan) achieves a maximum serum concentration of77% of the administered dose. The drug is 60% bound inthe plasma. It is extensively metabolized by CYP3A4, andseven metabolites have been identified. Oral bioavailabilityis good, with a tmax of 0.8±0.3 hour. The half-life ofelimination is 1.8 hour, and the elimination products aredetectable in feces and urine. Indinavir also causes dyslipidemia.The available dosage forms are capsules of 200 mg,333 mg, and 400 mg.
Pharmaceutical Applications
A synthetic compound formulated as the sulfate for oral administration.
Pharmacokinetics
Indinavir is a protease inhibitor with activity against Human Immunodeficiency Virus Type 1 (HIV-1). Protease inhibitors block the part of HIV called protease. HIV-1 protease is an enzyme required for the proteolytic cleavage of the viral polyprotein precursors into the individual functional proteins found in infectious HIV-1. Indinavir binds to the protease active site and inhibits the activity of the enzyme. This inhibition prevents cleavage of the viral polyproteins resulting in the formation of immature non-infectious viral particles. Protease inhibitors are almost always used in combination with at least two other anti-HIV drugs.
Pharmacokinetics
Oral absorption: c. 65%v
Cmax 800 mg thrice daily: c. 8.97 mg/L
Cmin 800 mg thrice daily: c. 0.15 mg/L
Plasma half-life: c. 2 h
Volume of distribution: c. 0.4–1.74 L/kg
Plasma protein binding: c. 60%
Absorption and distribution
It is rapidly absorbed and not significantly affected by intake with food. Distribution in the body has not been fully characterized. It penetrates well into the CNS. The semen:plasma ratio is 1.9. It is distributed into breast milk.
Metabolism and excretion
Seven major metabolites have been described, including a glucuronide conjugate and six oxidative metabolites. Around 83% of the dose is recovered in feces and 18% in urine, 10% as unchanged drug. The effect of renal impairment has not been studied. It should be used with caution in the presence of hepatic impairment, particularly if severe.
Clinical Use
Treatment of adult HIV infection (in combination with other antiretroviral drugs)
Side Effects
The principal side effect is nephrolithiasis, including flank pain
with or without hematuria. There is good evidence that indinavir
directly causes nephrolithiasis as a result of crystallization
in the urinary tract. Indirect hyperbilirubinemia occurs in
about 10% of patients associated with inhibition of bilirubinconjugating
activity occurring as a result of competitive inhibition
of uridine diphosphate (UDP)-glucuronosyltransferase.
Ritonavir-boosted indinavir is associated with a dyslipidemia
profile characteristic of those treated with other
protease
inhibitors boosted with a 200 mg dose of ritonavir
per day. Insulin resistance and hyperglycemia have also been
associated with ritonavir-boosted indinavir.
Drug interactions
Potentially hazardous interactions with other drugs
Anti-arrhythmics: possibly increased amiodarone
and flecainide concentration - avoid.
Antibacterials: rifampicin increases metabolism
- avoid concomitant use; increased rifabutin
concentration - avoid; avoid with telithromycin in
severe renal and hepatic failure.
Anticoagulants: avoid with apixaban and
rivaroxaban.
Antidepressants: concentration reduced by St John’s
wort - avoid.
Antiepileptics: concentration possibly reduced
by carbamazepine, fosphenytoin, phenytoin,
primidone and phenobarbital, also concentration
of carbamazepine, fosphenytoin and phenytoin
increased.
Antifungals: itraconazole and ketoconazole inhibits
metabolism - reduce dose of indinavir to 600 mg
every 8 hours.
Antimalarials: use artemether/lumefantrine with
caution; possibly increased quinine concentration.
Antipsychotics: possibly increased risk of ventricular
arrhythmias with pimozide - avoid; possibly inhibits
aripiprazole metabolism - reduce aripiprazole
dose; possibly increases lurasidone and quetiapine
concentration - avoid.
Antivirals: avoid with atazanavir; concentration
reduced by efavirenz, nevirapine and possibly
etravirine, avoid with etravirine; concentration of
both drugs increased with darunavir; concentration
of maraviroc increased, consider reducing maraviroc
dose; concentration increased by ritonavir; saquinavir
concentration increased.
Anxiolytics and hypnotics: increased risk of
prolonged sedation with alprazolam and midazolam
- avoid.
Avanafil: concentration of avanafil possibly increased
- avoid.
Ciclosporin: concentration of ciclosporin increased.
Colchicine: possibly increases risk of colchicine
toxicity, avoid in hepatic or renal impairment.
Cytotoxics: possibly increases concentration of
axitinib, reduce dose of axitinib; possibly increases
bosutinib, cabazitaxel and docetaxel concentration
- avoid or reduce dose of bosutinib, cabazitaxel
and docetaxel; possibly increases concentration of
crizotinib and everolimus - avoid; possibly increases
ibrutinib concentration, reduce dose of ibrutinib;
avoid with olaparib and pazopanib; reduce dose of
ruxolitinib.
Ergot alkaloids: risk of ergotism - avoid.
Guanfacine: possibly increases guanfacine
concentration, halve dose of guanfacine.
5HT1 agonists: concentration of eletriptan increased
- avoid.
Lipid-regulating drugs: avoid with lomitapide
increased risk of myopathy with rosuvastatin and
simvastatin - avoid; and possibly with atorvastatin.
Naloxegol: possibly increases naloxegol concentration
- avoid.
Orlistat: absorption possibly reduced by orlistat.
Ranolazine: possibly increases ranolazine
concentration - avoid.
Sildenafil: concentration of sildenafil increased -
reduce initial sildenafil dose.
Vardenafil: concentration of vardenafil increased -
avoid.
Metabolism
Hepatic. Seven metabolites have been identified, one glucuronide conjugate and six oxidative metabolites. In vitro studies indicate that cytochrome P-450 3A4 (CYP3A4) is the major enzyme responsible for formation of the oxidative metabolites.
Metabolism
The usual oral dose for indinavir alone or in combination with other antiviral agents is one 800 mg capsule every 8 hours. The drug is well absorbed if given on an empty stomach or 1 hour before or 2 hours after a light meal with water. The dose is reduced to 600 mg every 8 hours if given concurrently with ketoconazole. Indinavir activity is increased when combined with RT inhibitors.
Properties of Indinavir
| Melting point: | 153-154°; mp 167.5-168° |
| Boiling point: | 877.9±65.0 °C(Predicted) |
| alpha | D22 +24.1° (c = 0.0133 in chloroform) |
| Density | 1.25±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| pka | pKa 3.8/6.2(H2O,t undefined,Iundefined) (Uncertain) |
| Water Solubility | 70mg/L(temperature not stated) |
Safety information for Indinavir
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Indinavir
Indinavir manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
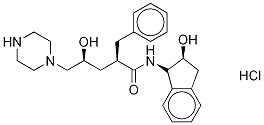
![[1(1S,2R),5(S)]-2,3,5-Trideoxy-N-(2,3-dihydro-2-hydroxy-1H-inden-1-yl)-5-[2-[[(1,1-dimethylethyl)amino]carbonyl]-1-piperazinyl]-2-(phenylmethyl)-D-erythro-pentonamide](https://img.chemicalbook.in/CAS/GIF/150323-38-9.gif)
![N-[N,O-ISOPROPYLIDENE-(2R)-HYDROXY INDAN-(1S)-YL]-(2R)-BENZYL-(4S,5)-EPOXY PENTANAMIDE](https://img.chemicalbook.in/CAS/GIF/158512-24-4.gif)
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1