Imazalil
Synonym(s):1-[2-(Allyloxy)-2-(2,4-dichlorophenyl)ethyl]imidazole;Imazalil
- CAS NO.:35554-44-0
- Empirical Formula: C14H14Cl2N2O
- Molecular Weight: 297.18
- MDL number: MFCD00055331
- EINECS: 252-615-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
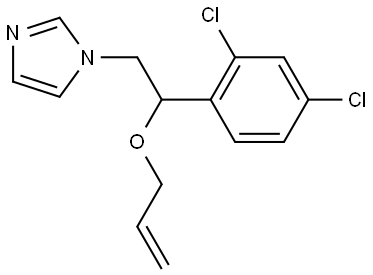
What is Imazalil?
Description
Imazalil is an imidazole fungicide that inhibits ergosterol biosynthesis. Imazalil inhibits the growth of various fungi in vitro including P. italicum, A. niger, U. maydis, B. alii, and C. cucumerinum in a pH-dependent manner (MICs = 0.005-2 μg/ml at pH 7). It inhibits S. cerevisiae, but not rat liver microsomal, cytochrome P450 enzymes (CYPs; IC50s = 0.088 and 80 μM, respectively), as well as aromatase CYP19 from human placental microsomes (IC50 = 0.34 μM). Imazalil activates the murine pregnane X receptor (PXR) in a concentration-dependent manner in a cell-based reporter assay. It increases hepatic CYP3A11 and CYP2B10 mRNA levels in mice when administered at a dose of 100 mg/kg. Imazalil also increases Ki-67-positive nuclei in liver sections and hepatic MCM2 mRNA levels, markers of cell proliferation, in mice when co-administered with the murine constitutive androstane receptor (mCAR) agonist TCPOBOP . Formulations containing imazalil have been used to control fungal infection in agriculture.
Description
The Uses of Imazalil
Imazalil is a systemic fungicide with protective and curative action. It is used for the control of a wide range of fungal diseases on fruit, vegetables and ornamentals, powdery mildew on roses and storage diseases of citrus fruit, pome fruit, bananas and seed potatoes. It is also used as a seed dressing, for control of diseases of cereals (particularly Fusarium and Helminthosporium spp.), and it is particularly active against benzimidazole-resistant strains of plant-pathogenic fungi.
The Uses of Imazalil
antifungal
The Uses of Imazalil
As a disinfectant for stable and kennel equipment; experimentaly as an agricultural fungicide.
What are the applications of Application
Imazalil is an antifungal agent that may have carcinogenic potential
Definition
ChEBI: 1-[2-(allyloxy)-2-(2,4-dichlorophenyl)ethyl]imidazole is a member of the class of imidazoles in which the hydrogen at position 1 is replaced by a 2-(allyloxy)-2-(2,4-dichlorophenyl)ethyl group. It is a member of imidazoles, an ether and a dichlorobenzene.
General Description
Slightly yellow to brown solidified oil. Non-corrosive. Used as a fungicide.
Air & Water Reactions
Water soluble.
Reactivity Profile
Imazalil is an imidazole derivative.
Safety Profile
Poison by ingestion and intraperitoneal routes. Experimental reproductive effects. A skin and eye irritant. When heated to decomposition it emits toxic fumes of Cland NOx.
Veterinary Drugs and Treatments
Although no dosage forms are currently commercially available for topical use in the USA, Enilconazole is used topically for treating dermatophytosis
in small animals and horses using compounded products. A commercially available topical rinse Imaverol? (Janssen) 10% is
available with canine, bovine and equine use labeling in many countries. Intranasal instillation of enilconazole after plaque debridement
has also been shown useful in treating nasal aspergillosis in small animals.
Use of topical enilconazole on cats with dermatophytosis is somewhat controversial as there are apparently no products with feline
labeling available in Europe or Canada. There are preliminary reports of safely and successfully using enilconazole on dermatophytic cats
in combination with oral itraconazole.
A topical product and a poultry environmental disinfectant product (Clinafarm EC?) is available in the USA. It is technically illegal to
use this product other than it is labeled; it is an EPA licensed product in the USA.
Metabolic pathway
Published information is available on the metabolism of imazalil in plants and soils. The principal metabolite in plants and soils is 1-[2-(2,4- dichlorophenyl)-2-hydroxyethyl]-1H-imidazole.
Degradation
Imazalil is very stable to hydrolysis in dilute acids and alkalis at room temperature, in the absence of light. It is also stable to light under normal storage conditions.
Properties of Imazalil
| Melting point: | 52.7°C |
| Boiling point: | >340°C |
| Density | 1.348 |
| vapor pressure | 1.58 x l0-4 Pa (20 °C) |
| refractive index | 1.5680 (estimate) |
| storage temp. | 2-8°C |
| solubility | Chloroform: Slightly Soluble; Methanol: Slightly Soluble |
| pka | 6.53 (weak base) |
| form | neat |
| form | Solid |
| color | Light yellow to yellow |
| Water Solubility | 0.018 g/100 mL |
| Merck | 13,3616 |
| BRN | 545683 |
| CAS DataBase Reference | 35554-44-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Imazalil(35554-44-0) |
| EPA Substance Registry System | Imazalil (35554-44-0) |
Safety information for Imazalil
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H318:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Imazalil
| InChIKey | PZBPKYOVPCNPJY-UHFFFAOYSA-N |
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid Fmoc-Val-Cit-PAB DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonateRelated products of tetrahydrofuran




You may like
-
 Imazalil CAS 35554-44-0View Details
Imazalil CAS 35554-44-0View Details
35554-44-0 -
 Imazalil CAS 35554-44-0View Details
Imazalil CAS 35554-44-0View Details
35554-44-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8