Idebenone
Synonym(s):2-(10-hydroxydecyl)-5,6-dimethoxy-3-methylcyclohexa-2,5-diene-1,4-dione
- CAS NO.:58186-27-9
- Empirical Formula: C19H30O5
- Molecular Weight: 338.44
- MDL number: MFCD00274552
- EINECS: 1308068-626-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:05
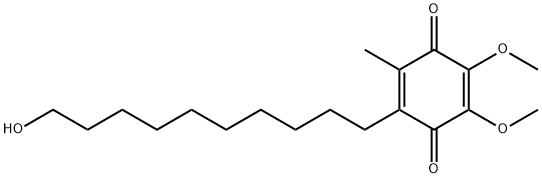
What is Idebenone?
Absorption
After oral administration, idebenone is rapidly absorbed. On repeat dosing, maximum plasma concentrations of idebenone are reached on average within 1 hour (median 0.67 h range: 0.33-2.00 h). Food increases the bioavailability of idebenone by approximately 5-7-fold and i therefore should always be administered with food .
Toxicity
The most commonly reported adverse reactions to idebenone are mild to moderate diarrhoea (usually not requiring the discontinuation of the treatment), nasopharyngitis, cough and back pain .
Description
Idebenone is an organic compound belonging to the quinone family, being similar to coenzyme Q-10. It is a kind of drug developed by Takeda Pharmaceutical Company for the treatment of Alzheimer’s disease and some other cognitive defects. However, these have been not very much progress associated with this indication. It is now also used for the treatment of Friedreich’s ataxia with a positive effect on cardiac hypertrophy and neurological function. However, this indication is only approved in Canada, not in Europe and US. It is now under investigation on its efficacy for the treatment of Duchenne muscular dystrophy, Leber’s hereditary optic neuropathy, mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes) as well as primary progressive multiple sclerosis. The efficacy of this drug still demands more evidences.
Chemical properties
An odorless yellow-orange crystalline solid. It is highly insoluble in water, but can be easily dissolved in chloroform, methanol, or absolute ethanol. It is also soluble in ethyl acetate, but insoluble in n-hexane.
Originator
Takeda (Japan)
The Uses of Idebenone
idebenone is an anti-oxidant capable of protecting the skin from a variety of free-radical attacks, including the formation of secondary chemicals that negatively affect skin physiology. It is said to improve intrinsic as well as extrinsic skin damage caused by freeradical formation. Idebenone is a synthetically manufactured form of coenzyme Q10 and has a smaller molecular structure. This allows it to penetrate the skin and apparently the cellular membrane. Clinical studies demonstrate a visible improvement in photodamaged skin, reduced skin roughness and dryness, decreased fine lines and wrinkles, and increased skin hydration. In addition, idebenone helps improve hyperpigmentation because its molecular structure is similar to that of hydroquinone. Although most of the associated benefits are seen primarily in the epidermis, some increase in dermal collagen has also been confirmed. Idebenone has been used for such health-related problems as Alzheimer’s and heart disease.
Indications
Idebenone is indicated for use by the European Medicines Agency (EMA) for the treatment of visual impairment in adolescent and adult patients with Leber’s Hereditary Optic Neuropathy (LHON). It is not currently approved for use by either the Food and Drug Administration (USA) or Health Canada .
What are the applications of Application
Idebenone is an antioxidant and neuroprotective agent
Background
Idebenone is a synthetic analogue of ubiquinone (also known as Coenzyme Q10), a vital cell antioxidant and essential component of the Electron Transport Chain (ETC). It has been proposed that by interacting with the ETC, idebenone increases ATP production required for mitochondrial function, reduces free radicals, inhibits lipid peroxidation, and consequently protects the lipid membrane and mitochondria from oxidative damage . More specifically, idebenone is thought to transfer electrons directly to complex III of the mitochondrial ETC, thereby circumventing complex I and restoring cellular energy (ATP) generation .
Due to its ability to reduce oxidative damage and improve ATP production, idebenone was originally investigated for its potential use in Alzheimer's Disease and other cognitivie disorders . Lack of improvement in cognitive function halted its production for these conditions, however it continues to be investigated for use in other conditions associated with mitochondrial damage.
Idebenone is currently only indicated for use by the European Medicines Agency (EMA) for the treatment of visual impairment in adolescent and adult patients with
Leber’s Hereditary Optic Neuropathy (LHON). LHON is a mitochondrially inherited degeneration of retinal ganglion cells, resulting in acute central vision loss. Due to its biochemical mode of action, it's thought that idebenone may re-activate viable-but-inactive retinal ganglion cells (RGCs) in LHON patients . It is not currently approved for use by either the Food and Drug Administration (USA) or Health Canada.
Definition
ChEBI: Idebenone is a member of the class of 1,4-benzoquinones which is substituted by methoxy groups at positions 2 and 3, by a methyl group at positions 5, and by a 10-hydroxydecyl group at positions 6. Initially developed for the treatment of Alzheimer's disease, benefits were modest; it was subsequently found to be of benefit for the symptomatic treatment of Friedreich's ataxia. It has a role as an antioxidant and a ferroptosis inhibitor. It is a primary alcohol and a member of 1,4-benzoquinones.
What are the applications of Application
Idebenone is ubiquinone derivative with protective effects against cerebral ischemia and cognition enhancer. It has been used:
chemotherapy drug
to treat mutant myocilin (mMYOC) cells for drug treatment assay
to validate C2C12 secondary cell screening assay
to treat obese mice, to test whether idebenone and CoQ10 bind directly to peroxisome proliferator-activated receptor (PPAR)LBDs, His-tagged PPARα, δ and γ LBDs
brand name
AVAN
General Description
Idebenone is a short-chain benzoquinone drug. It is a structural relative of ubiquinone (Coenzyme Q10) that protects the CNS from the effects of ischemia via improving brain metabolism. It is reported to be of potential use in the management of patients with cerebral apoplexy, cerebral arteriosclerosis and related disorders.
Biological Activity
Antioxidant and neuroprotective agent. Protects mitochondrial membranes against lipid peroxidation and blocks glutamate neurotoxicity in vitro and in vivo . Inhibits apoptosis of astrocytes via increased NGF production.
Biochem/physiol Actions
Idebenone is used in the treatment of visual impairment in adolescents and adults with Leber′s hereditary optic neuropathy (LHON). It serves as an antioxidant. It helps to guard the heart muscle against oxidative stress. A short-chain Coenzyme Q analog that enhances superoxide formation, presumably by mediating electron transfer from N2 to oxygen.
Side Effects
The most common adverse reactions to Idebenone are mild to moderate diarrhoea (usually without the need to stop treatment), nasopharyngitis, cough and back pain.
The following adverse reactions emerging from clinical trials in LHON patients or reported postmarketing in other indications are tabulated below. Frequency groupings are defined to the following
convention: very common (≥1/10), common (≥1/100 to<1/10), not known (cannot be estimated from
the available data).

Metabolism
Metabolism occurs by means of oxidative shortening of the side chain and by reduction of the quinone ring and conjugation to glucuronides and sulphates. Idebenone shows a high first pass metabolism resulting in conjugates of idebenone (glucuronides and sulphates (IDE-C)) and the Phase I metabolites QS10, QS6, and QS4 as well as their corresponding Phase II metabolites (glucuronides and sulphates (QS10+QS10-C, QS6+QS6-C, QS4+QS4-C)). The main metabolites in plasma are IDE-C and QS4+QS4-C .
Mode of action
The mechanism of action of idebenone involves its antioxidant properties and ability to act as a mitochondrial electron carrier. Idebenone overcomes mitochondrial complex I respiratory chain deficiency in patients with LHON by transferring electrons directly to mitochondrial complex III (by-passing complex I), thereby restoring cellular energy (ATP) production and re-activating inactive-but-viable retinal ganglion cells, which ultimately prevents further vision loss and promotes vision recovery.
PID27071925
References
1) Zs-Nagy (1990) Chemistry, toxicology, pharmacology and pharmacokinetics of idebenone: a review; Gerontol. Geriatr., 11, 177
2) Civenni et al. (1999) Inhibitory effect of the neuroprotetive agent idebenone on arachidonic acid metabolism in astrocytes; Eur. J. Pharmacol., 370 161
3) Takuma et al. (2000) CV-2619 protects cultured astrocytes against reperfusion injury via nerve growth factor production; Eur. J. Pharmacol., 406 333
4) Gerhardt et al. (2011) Idebenone and resveratrol extend lifespan and improve motor function of HtrA2 knockout mice; PloS One, 6 e28855
Properties of Idebenone
| Melting point: | 52-550C |
| Boiling point: | 497.3±45.0 °C(Predicted) |
| Density | 1.08±0.1 g/cm3(Predicted) |
| storage temp. | room temp |
| solubility | Soluble in DMSO (up to 25 mg/ml). |
| form | neat |
| pka | 15.20±0.10(Predicted) |
| form | Solid |
| color | Orange |
| Merck | 14,4888 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| InChI | InChI=1S/C19H30O5/c1-14-15(12-10-8-6-4-5-7-9-11-13-20)17(22)19(24-3)18(23-2)16(14)21/h20H,4-13H2,1-3H3 |
| CAS DataBase Reference | 58186-27-9(CAS DataBase Reference) |
Safety information for Idebenone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Idebenone
| InChIKey | JGPMMRGNQUBGND-UHFFFAOYSA-N |
| SMILES | C1(=O)C(OC)=C(OC)C(=O)C(C)=C1CCCCCCCCCCO |
Idebenone manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran






You may like
-
 Idebenone 98% CAS 58186-27-9View Details
Idebenone 98% CAS 58186-27-9View Details
58186-27-9 -
 Idebenone CAS 58186-27-9View Details
Idebenone CAS 58186-27-9View Details
58186-27-9 -
 Idebenone CAS 58186-27-9View Details
Idebenone CAS 58186-27-9View Details
58186-27-9 -
 Idebenone CAS 58186-27-9View Details
Idebenone CAS 58186-27-9View Details
58186-27-9 -
 CAS:58186-27-9 Idebenone 99% Idebenone PowderView Details
CAS:58186-27-9 Idebenone 99% Idebenone PowderView Details
58186-27-9 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
