HYDROFLUMETHIAZIDE
Synonym(s):3,4-Dihydro-6-(trifluoromethyl)-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide
- CAS NO.:135-09-1
- Empirical Formula: C8H8F3N3O4S2
- Molecular Weight: 331.29
- MDL number: MFCD00057316
- EINECS: 205-173-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-21 22:16:43
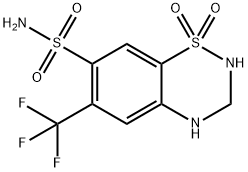
What is HYDROFLUMETHIAZIDE?
Absorption
Hydroflumethiazide is incompletely but fairly rapidly absorbed from the gastrointestinal tract
Toxicity
Overdoses lead to diuresis, lethargy progressing to coma, with minimal cardiorespiratory depression and with or without significant serum electrolyte changes or dehydration.
Chemical properties
White Solid
Originator
Saluron,Bristol,US,1959
The Uses of HYDROFLUMETHIAZIDE
Labelled Hydroflumethiazide. Antihypertensive; diuretic.
Indications
Used as adjunctive therapy in edema associated with congestive heart failure, hepatic cirrhosis, and corticosteroid and estrogen therapy. Also used in the management of hypertension either as the sole therapeutic agent or to enhance the effect of other antihypertensive drugs in the more severe forms of hypertension.
What are the applications of Application
Hydroflumethiazide is an anti-hypertensive and a diuretic
Background
A thiazide diuretic with actions and uses similar to those of hydrochlorothiazide. (From Martindale, The Extra Pharmacopoeia, 30th ed, p822)
Definition
ChEBI: Hydroflumethiazide is a benzothiadiazine consisting of a 3,4-dihydro-HH-1,2,4-benzothiadiazine bicyclic system dioxygenated on sulfur and carrying trifluoromethyl and aminosulfonyl groups at positions 6 and 7 respectively. A diuretic with actions and uses similar to those of hydrochlorothiazide. It has a role as a diuretic and an antihypertensive agent. It is a benzothiadiazine and a thiazide.
Manufacturing Process
(a) Preparation of 5-Trifluoromethylaniline-2,4-Disulfonyl Chloride: 113 ml of
chlorosulfonic acid was cooled in an ice-bath, and to the acid was added
dropwise while stirring 26.6 grams of α,α,α-trifluoro-m-toluidine. 105 grams of
sodium chloride was added during 1 to 2 hours, whereafter the temperature
of the reaction mixture was raised slowly to 150° to 160°C, which
temperature was maintained for 3 hours. After cooling the mixture, ice-cooled
water was added, whereby 5-trifluoromethylaniline-2,4-disulfonyl chloride
separated out from the mixture.
(b) Preparation of 5-Trifluoromethyl-2,4-Disulfamylaniline: The 5-
trifluoromethylaniline-2,4-disulfonyl chloride obtained in step (a) was taken up
in ether and the ether solution dried with magnesium sulfate. The ether was
removed from the solution by distillation, the residue was cooled to 0°C and
60 ml of ice-cooled, concentrated ammonia water was added while stirring.
The solution was then heated for one hour on a steam bath and evaporated in
vacuo to crystallization. The crystallized product was 5-trifluoromethyl-2,4-
disulfamylaniline, which was filtered off, washed with water and dried in a
vacuum exsiccator over phosphorus pentoxide. After recrystallization from a
mixture of 30% ethanol and 70% water the compound had a MP of 247° to
248°C.
(e) Preparation of 6-Trifluoromethyl-7-Sulfamyl-3,4-Dihydro-1,2,4-
Benzothiadiazine-1,1-Dioxide: 3.2 grams of 5-trifluoromethyl-2,4-
disulfamylaniline was added to a solution of 0.33 gram of paraformaldehyde in
25 ml of methyl Cellosolve (2-methoxy ethanol) together with a catalytic
amount of p-toluenesulfonic acid, and the mixture was boiled with reflux for 5
hours. The solvent was then distilled off in vacuo, and the residue triturated
with 30 ml of ethyl acetate. 6-trifluoromethyl-7-sulfamyl-3,4-dihydro-1,2,4-
benzothiadiazine-1,1-dioxide crystallized out. After recrystallization from methanol/water the substance had a MP of 272° to 273°C.
Therapeutic Function
Diuretic, Antihypertensive
Pharmacokinetics
Hydroflumethiazide is an oral thiazide used to treat hypertension and edema. High blood pressure adds to the workload of the heart and arteries. If it continues for a long time, the heart and arteries may not function properly. This can damage the blood vessels of the brain, heart, and kidneys, resulting in a stroke, heart failure, or kidney failure. High blood pressure may also increase the risk of heart attacks. Like other thiazides, Hydroflumethiazide promotes water loss from the body (diuretics). Thiazides inhibit Na+/Cl- reabsorption from the distal convoluted tubules in the kidneys. Thiazides also cause loss of potassium and an increase in serum uric acid. Thiazides are often used to treat hypertension, but their hypotensive effects are not necessarily due to their diuretic activity. Thiazides have been shown to prevent hypertension-related morbidity and mortality although the mechanism is not fully understood. Thiazides cause vasodilation by activating calcium-activated potassium channels (large conductance) in vascular smooth muscles and inhibiting various carbonic anhydrases in vascular tissue.
Metabolism
Essentially unchanged
Properties of HYDROFLUMETHIAZIDE
| Melting point: | 272-273 |
| Boiling point: | 531.6±60.0 °C(Predicted) |
| Density | 1.5955 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| solubility | DMSO (Slightly), Methanol (Sparingly) |
| form | Solid |
| pka | 8.9, 8.2(at 25℃) |
| color | White |
| Water Solubility | 329.9mg/L(room temperature) |
| EPA Substance Registry System | 2H-1,2,4-Benzothiadiazine-7-sulfonamide, 3,4-dihydro-6-(trifluoromethyl)-, 1,1-dioxide (135-09-1) |
Safety information for HYDROFLUMETHIAZIDE
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H334:Sensitisation, respiratory |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P284:Wear respiratory protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P342+P311:IF experiencing respiratory symptoms: call a POISON CENTER or doctor/physician. |
Computed Descriptors for HYDROFLUMETHIAZIDE
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Hydroflumethiazide CAS 135-09-1View Details
Hydroflumethiazide CAS 135-09-1View Details
135-09-1 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
