HUMAN FIBRINOGEN
Synonym(s):Coagulation Factor I;Factor I;Fibrinogen, Bovine Plasma;Fibrinogen, Human Plasma;Fibrinogen, Plasminogen-Depleted, Human Plasma
- CAS NO.:9001-32-5
- Empirical Formula: C6H14O6
- Molecular Weight: 182.17176
- MDL number: MFCD00131060
- EINECS: 232-598-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-25 11:31:46
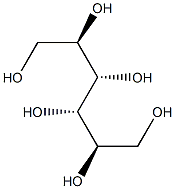
What is HUMAN FIBRINOGEN?
Chemical properties
White or grayish amorphous substance.
The Uses of HUMAN FIBRINOGEN
Diagnostic aid (vascular patency); radioactive agent.
The Uses of HUMAN FIBRINOGEN
A soluble plasma glycoprotein, produced by the liver, that is converted by thrombin into fibrin during the blood coagulation process. Fibrinogen is a natural blood protein involved in the coagulation process. It is used instead of traditional sources of fibrinogen such as the blood products, fresh frozen plasma and cryoprecipitate. It helps in blood clot formation.
The Uses of HUMAN FIBRINOGEN
Fibrinogen from rat plasma has been used:
- in the preparation of growth factor solutions
- to apply on discontinuous SDS-polyacrylamide gels for the detection of IgG (immunoglobulins) type antibodies by immunoblot analysis
- to mix with cell suspension to form a clot and retain the cells for avoiding its dispersion during transplantation
Definition
A sterile fraction of normal human blood plasma, dried from the frozen state. In solution, it has the property of being converted into insoluble fibrin when thrombin is added. It is an essential factor in the blood-clotting mechanism.
General Description
Fibrinogen, or Factor I, is a blood protein that is involved in clotting and is converted to fibrin by thrombin. Fibrinogen has an approximate molecular weight of 340 kDa
Biochem/physiol Actions
Fibrinogen is an acute phase protein that is part of the coagulation cascade of proteins. The end result of the cascade is the production of thrombin that converts fibrinogen to fibrin. Thrombin rapidly proteolyses fibrinogen, releasing fibrinopeptide A. The loss of this small peptide is not sufficient to make the resulting fibrin molecule insoluble, but it tends to form complexes with adjacent fibrin and fibrinogen molecules. Thrombin then cleaves a second peptide, fibrinopeptide B, from fibrin and the fibrin monomers formed then polymerize spontaneously to form an insoluble gel. The polymerized fibrin is held together by noncovalent and electrostatic forces and stabilized by the transamidating enzyme, factor XIIIa, that is produced by the action of thrombin on factor XIII. The insoluble fibrin aggregates (clots) and aggregated platelets then block the damaged blood vessel and prevent further bleeding. The amount of fibrinogen in the plasma can serve as a nonspecific indicator of whether or not an inflammatory process is present in the body. Fibrinogen from any mammalian source will be cleaved by thrombin from any mammalian source.
Purification Methods
A likely impurity is plasminogen. It is purified by glycine precipitation [Mosesson & Sherry Biochemistry 5 2829 1966] to obtain fractions 1-2, then further purified [Blomb.ck & Blomb.ck Arkiv Kemi 10 415 1956] and contaminating plasminogen is removed by passage through a lysine-Sepharose column. Such preparations are at least 95% clottable as determined by Mosesson and Sherry's method (above ref.) in which the OD280 is measured before and after clotting with 5 Units/mL of thrombin (> 3000U/mg). All fibrinogen preparations are treated with calf intestinal alkaline phosphatase to convert any fibrinogen peptide-AP to fibrinogen peptide-A by removing serine-bound phosphate. Solutions are then lyophilised and stored at -20o. [Higgins & Shafer J Biol Chem 256 12013 1981.] It is sparingly soluble in H2O. Aqueous solutions are viscous with isoelectric point at pH 5.5. It is readily denatured by heating above 56o or by chemical agents, e.g. salicylaldehyde, naphthoquinone sulfonates, ninhydrin or alloxan. [Edsall et al. J Am Chem Soc 69 2731 1947, Purification: Cama et al. Naturwissenschaften 48 574 1961, Lorand & Middlebrook Science 118 515 1953, cf. Fuller in Methods Enzymol 163 474 1988.] For plasminogen-deficient fibrinogen from blood plasma, the anticoagulated blood is centrifuged and the plasma is frozen and washed with saline solution. It is treated with charcoal, freeze-thawed and dialysed versus Tris/NaCl buffer. [Maxwell & Nikel Biochemical Preparations 12 16 1968.]
Properties of HUMAN FIBRINOGEN
| storage temp. | -20°C |
| solubility | 0.9% NaCl: soluble |
| form | powder |
| color | white |
| EPA Substance Registry System | Fibrinogens (9001-32-5) |
Safety information for HUMAN FIBRINOGEN
Computed Descriptors for HUMAN FIBRINOGEN
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran







You may like
-
 Fibrinogen from rat plasma CAS 9001-32-5View Details
Fibrinogen from rat plasma CAS 9001-32-5View Details
9001-32-5 -
 Fibrinogen, Bovine Plasma CAS 9001-32-5View Details
Fibrinogen, Bovine Plasma CAS 9001-32-5View Details
9001-32-5 -
 Fibrinogen from human plasma CAS 9001-32-5View Details
Fibrinogen from human plasma CAS 9001-32-5View Details
9001-32-5 -
 Fibrinogen, Plasminogen-Depleted, Human Plasma CAS 9001-32-5View Details
Fibrinogen, Plasminogen-Depleted, Human Plasma CAS 9001-32-5View Details
9001-32-5 -
 Fibrinogen from bovine plasma CAS 9001-32-5View Details
Fibrinogen from bovine plasma CAS 9001-32-5View Details
9001-32-5 -
 Fibrinogen from human plasma CAS 9001-32-5View Details
Fibrinogen from human plasma CAS 9001-32-5View Details
9001-32-5 -
 Fibrinogen, Human Plasma CAS 9001-32-5View Details
Fibrinogen, Human Plasma CAS 9001-32-5View Details
9001-32-5 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
