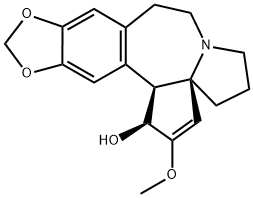
Homoharringtonine
Synonym(s):Omacetaxine mepesuccinate;HHT;Ceflatonin;Cephalotaxine 4-methyl (2R)-2-hydroxy-2-(4-hydroxy-4-methylpentyl)butanedioate;Myelostat
- CAS NO.:26833-87-4
- Empirical Formula: C29H39NO9
- Molecular Weight: 545.63
- MDL number: MFCD05618221
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-27 15:38:00
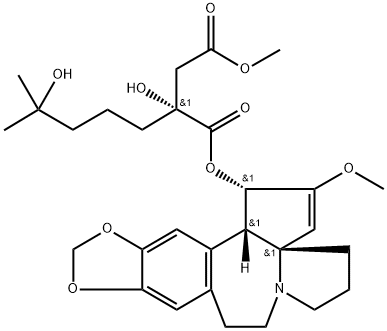
What is Homoharringtonine?
Absorption
Homoharringtonine absorption was not quantified, but maximum concentration is reached after about 30 mins.
Toxicity
The most severe adverse effects after homoharringtonine administration are myelosuppression, bleeding, hyperglycemia, and fetal harm.
Description
Omacetaxine mepesuccinate (also known as homoharringtonine) was approved by the US FDA in October 2012 for the treatment of patients with chronic or accelerated phase chronic myeloid leukemia (CML) with resistance or intolerance to at least two tyrosine kinase inhibitors (TKIs). Omacetaxine is a protein synthesis inhibitor that was studied in the 1970s for the treatment of acute myeloid leukemia (AML) and in the 1990s for CML. Emergence of resistance to first- and second-generation TKIs has lead to renewed interest in omacetaxine due to its differentiated mode of action. Omacetaxine acts on the initial step of protein translation andresults in the rapid loss of a number of short-lived proteins that regulate proliferation and cell survival. Omacetaxine induces apoptosis and shows in vitro activity in anumberof leukemia cell lines andinmurine leukemiamodels. Omacetaxineis a naturally occurring alkaloid isolated from Cephalotaxus coniferous shrubs that are indigenous to Asia. Extracts of the bark have been used by practitioners of traditional Chinese medicine for the treatment of cancer. Although omacetaxine could be isolated directly from bark and roots, a more efficient approach is semi-synthesis by esterification of the abundant biosynthetic precursor cephalotaxine, which can be extracted from leaves rather than nonrenewable sources. Esterification is carried out with an activated ester in which the diol side-chain is protected as a tetrahydropyran; after ester formation, the diol is released in two steps under mild conditions.
Description
Homoharringtonine is an alkaloid originally isolated from C. harringtonia and a homolog of harringtonine that has diverse biological activities including protein synthesis inhibitory, antiviral, antiparasitic, and anticancer properties. Homoharringtonine inhibits the chain elongation phase of translation in eukaryotes. It inhibits diphenylalanine formation by rabbit reticulocyte and human placental ribosomes in cell-free assays and binds to human 80S ribosomes (Kd = 39 nM). Homoharringtonine is active against coronaviruses, reducing the viral load in vitro and in vivo and prevents severe symptoms in porcine and chicken models of porcine epidemic diarrhea virus (PEDV) and Newcastle disease virus (NDV), respectively. It reduces the infectious virus yield and viral RNA copy numbers in the culture supernatant of Vero E6 cells infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (EC50s = 2.55 and 2.14 μM, respectively). It also inhibits the growth of P. falciparum in cultured in human erythrocytes (IC50 = 4 nM). Homoharringtonine is cytotoxic and inhibits the proliferation of Jurkat acute T cell leukemia (IC50 = 9 nM) and K562 chronic myelogenous leukemia (CML) cells (IC50 = 408 μg/ml). In vivo, it decreases the number of peripheral leukemia stem cells and increases survival in CML and B cell acute lymphoblastic leukemia (B-ALL) mouse models when administered at a dose of 0.5 mg/kg. Formulations containing homoharringtonine have been used in the treatment of CML in patients with resistance and/or intolerance to two or more tyrosine kinase inhibitors.
Chemical properties
Off-white Cryst
Physical properties
Appearance: an almost white or pale yellow crystalline powder or an amorphous friable solid. It has the hygroscopic nature. It darkens on exposure to light. Solubility: easily soluble in chloroform, ethanol and methanol, and slightly soluble in ether and water. Melting point: 143–147?°C.
Originator
Chinese Academy of Medical Sciences/ChemGenex (China/United States)
History
In China, especially in Fujian, etc., doctors have treated tumor with Cephalotaxus harringtonia long ago. In the early 1970s, American scientists Powell et?al. isolated and identified the alkaloids of the Cephalotaxus plant and studied its antitumor activity. One type of the alkaloids (Cephalotaxine ester, including harringtonine, homoharringtonine, isoharringtonine, deoxyharringtonine, and pseudodeoxyharringtonine) was found to have the effect of inhibiting the proliferation of mouse leukemia cells . Chinese scientists also isolated a large number of harringtonine and homoharringtonine and used them to treat leukemia. Then, the United States and other developed countries’ scientists carried out the Phase I and II clinical research and toxicology research of homoharringtonine.
The Uses of Homoharringtonine
Homoharringtonine has been used to check the cytotoxic activity against carfilzomib-resistant derivative of the LP-1 multiple myeloma (MM) cell line (LP-1/Cfz) and is used as a translation-inhibiting drug.
The Uses of Homoharringtonine
Homoharringtonine (HHT) combined with some botanical drugs could induce cancer cells to resemble normal cells. HHT was prepared by a semi-synthetic method from Cephalotaxine, a major alkaloid of Cepahlotaxus species through the formation of a-ketoes
Background
Omacetaxine mepesuccinate (formerly known as HHT or Homoharringtonine), is a cephalotaxine ester and protein synthesis inhibitor with established clinical activity as a single agent in hematological malignancies. Omacetaxine mepesuccinate is synthesized from cephalotaxine, which is an extract from the leaves of the plant, Cephalotaxus species. In October 2005, omacetaxine mepesuccinate received Orphan Drug designation from the EMEA for the treatment of chronic myeloid leukemia (CML). Then in March 2006, it received Orphan Drug status from the FDA for the treatment of CML. In November 2006, omacetaxine mepesuccinate, for the treatment of CML, was granted Fast Track designation by the FDA. Most recently, in October 2012, omacetaxine mepesuccinate was marketed under the brand name Synribo and FDA approved for patients who are intolerant and/or resistant to two or more tyrosine kinase inhibitors used to treat accelerated or chronic phase CML.
Indications
Used in patients who are intolerant and/or resistant to two or more tyrosine kinase inhibitors used to treat accelerated or chronic phase CML.
What are the applications of Application
Homoharringtonine is an inhibitor of diphenylalanine formation & acetylphenylalanyl-puromycin
Definition
ChEBI: A cephalotaxine-derived alkaloid ester obtained from Cephalotaxus harringtonia; used for the treatment of chronic or accelerated phase chronic myeloid leukaemia.
Indications
This product is recorded in the Pharmacopoeia of the People’s Republic of China (2015).
Its main dosage form is homoharringtonine injection, which is mainly used for
the treatment of chronic myelocytic leukemia and acute myeloid leukemia.
brand name
Synribo
Biological Activity
Inhibitor of protein synthesis. Blocks elongation phase of translation by binding to the 60-S ribosome subunit. Antileukemic.
Biochem/physiol Actions
Homoharringtonine is a cephalotaxine ester that is also known as (HHT; 4-methyl (2R)-2-hydroxy-2-(4-hydroxy-4-methylpentyl)butanedioate). In gefitinib-resistant lung cancer cells, homoharringtonine promotes apoptosis and prevents signal transducer and activator of transcription 3 (STAT3) through IL-6 (interleukin-6) /JAK1 (janus kinase 1)/STAT3 signal pathway. It plays an important role in the treatment of malaria.
Pharmacokinetics
The pharmacodynamics of homoharringtonine is not fully understood. It is known that homoharringtonine is involved with protein synthesis inhibition and this leads to its antineoplastic activity.
Pharmacology
Induce apoptosis: Homoharringtonine was found to activate HL-60 cell apoptosis through caspase-3 mediated Bcl-2-Bax, -MAPK pathway .
3. Induce cell differentiation: Homoharringtonine could induce HL-60 cell differentiation by downregulating the expression of CD44 gene, thereby increasing p27 and p21 expression and inhibition of cyclin E activity.
Structure-Activity Relationship (SAR) The methyl acetate group of 2′ position in the C-3 acyl side chain is an active essential group. R group plays a role in regulating the polarity of the molecule, and the size of the R group can affect the molecular activity. The olefinic carbon instead of the 2′ position chiral carbon is still active .
Anticancer Research
This compound is isolated from Cephalotaxus harringtonia. A racemic mixture ofharringtonine and homoharringtonine is used for acute and chronic myelogenousleukemia (Shoeb 2006).
Clinical Use
Synribo® (Omacetaxine mepesuccinate) was approved by the FDA for the treatment of adult patients with chronic or accelerated phase chronic myeloid leukemia (CML) exhibiting resistance or intolerance to tyrosine kinase inhibitors (TKI’s). Omacetaxine mepesuccinate inhibits protein synthesis and prevents aminoacyl-tRNA binding during the elongation phase and targets myeloma-promoting molecules Mcl-1, XIAP, and β-catenin, which are particularly important in the survival of myeloma cells. Omacetaxine mepesuccinate is also known as homoharringtonine, an alkaloid originally discovered and structurally identified from Cephalotaxus harringtonia, which occurs naturally in Japan and eastern Asia.
Synthesis
Because of its leukemic activity and interesting chemical structure,
the core and ester side chains of the cephalotaxine alkaloids have been the focus of numerous synthetic
studies. However, large-scale production often utilizes a semisynthetic route which relies upon
cephalataxine (CET) derived from natural sources coupled with a synthetically obtained ester side
chain. The challenges associated with direct esterification of cephalotaxine with the
homoharringtonine and other related ester side chains are the basis of ongoing research aimed at
identification of improved side-chain coupling methods. The most likely process-scale synthetic
route features the coupling of the homoharringtonine side chain with the cephalotaxine core, and a
subsequent conversion of the |á-hydroxy moiety to a bridged heterocyclic species followed by ring
opening after coupling to give the active homoharringtonine product, and this is described in the scheme below.
The method for large scale synthesis of homoharringtonine begins with derivatization of commercial
5-bromo-2-methyl-pent-2-ene (100) with diethyl oxalate and the pre-formed enolate of methyl acetate
(101), generating diester 102 in 50% overall yield. Acid-promoted pyran formation,
followed by universal ester saponification and selective re-esterification provided the desired racemic
pyran acid 103. Activation of acid 103 with 2,4,6-trichlorobenzoyl chloride (104) and subsequent addition of quinine (105) led to a mixture of diastereomers 106a/106b (1:1) in 84% yield, which were
separable by chromatographic methods. Diastereomer 106a was then carried on to the synthesis of
homoharringtonine (Omacetaxine mepesuccinate) as described in the scheme below.
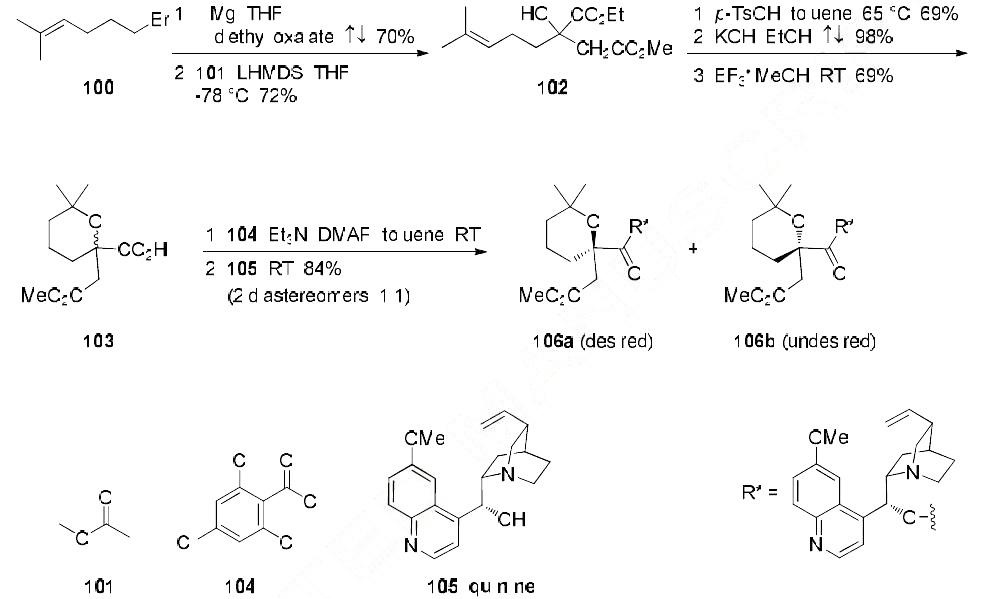
From isomer 106a, hydrogenolysis with Pd/C and H2 provided acid 107 in 50% yield. Activation of 107 with 2,4,6-trichlorobenzoyl chloride 104 followed by addition of cephalotaxine (CET) (108) provided the desired cephalotaxine-coupled product 109 in 43% yield. Sequential treatment with HBr/HOAc and 5% aqueous NaHCO3 completed the synthesis, providing omacetaxine mepesuccinate (XVII) in 41% yield (over two steps).
Metabolism
Homoharringtonine has undergoes little hepatic metabolism and is mostly metabolized to 4’-DMHHT by plasma esterase hydrolysis.
Storage
+4°C
Properties of Homoharringtonine
| Melting point: | 144-146 C |
| Boiling point: | 619.03°C (rough estimate) |
| Density | 1.2395 (rough estimate) |
| refractive index | 1.6290 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMSO: soluble20mg/mL, clear |
| form | powder |
| pka | 11.60±0.29(Predicted) |
| color | white to beige |
| optical activity | [α]/D -120 to -140°, c = 1 in chloroform-d |
Safety information for Homoharringtonine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| Precautionary Statement Codes |
P262:Do not get in eyes, on skin, or on clothing. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Homoharringtonine
| InChIKey | DJIVDDPFKDEQIR-XSEHADPMSA-N |
Homoharringtonine manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran





You may like
-
 Homoharringtonine >98% (HPLC) CAS 26833-87-4View Details
Homoharringtonine >98% (HPLC) CAS 26833-87-4View Details
26833-87-4 -
 Homoharringtonine CAS 26833-87-4View Details
Homoharringtonine CAS 26833-87-4View Details
26833-87-4 -
 ANTI-ACVRL1 antibody produced in mouse CASView Details
ANTI-ACVRL1 antibody produced in mouse CASView Details -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
