HOLMIUM
Synonym(s):;Holmium;
- CAS NO.:7440-60-0
- Empirical Formula: Ho
- Molecular Weight: 164.93
- MDL number: MFCD00011049
- EINECS: 231-169-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
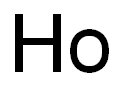
What is HOLMIUM?
Description
Holmium has the highest magnetic moment (10.6uB) of any naturally occurring element. Because of this it has been used to create the highest known magnetic fields by placing it within high strength magnets as a pole piece or magnetic flux concentrator.
This magnetic property also has value in Yttrium-Iron-Garnet (YIG) lasers for microwave equipment.
Holmium lasers emit at a human eye safe 2.08 microns allowing its use in a variety of medical and dental applications in both Yttrium-Aluminum-Garnet (YAG) and Yttrium-Lanthanum-Fluoride (YLF) solid state lasers. The wavelength allows for use in silica fibers designed for shorter wavelengths while still providing the cutting strength of longer wave length equipment.
Holmium is one of the colorants used for cubic zirconia and glass, providing yellow or red coloring.
Holmium Metal, is mainly used for making speciality alloys and superconductive materials. Holmium is used in Yttrium-Aluminum-Garnet (YAG) and Yttrium-Lanthanum-Fluoride (YLF) solid-state lasers found inmicrowave equipment (which are in turn found in a variety of medical and dental settings). Holmium lasers are used in medical, dental, and fiber-optical applications. Holmium is one of the colorants used for cubic zirconia and glass, providing yellow or red coloring. Holmium Metal can be further processed to various shapes of ingots, pieces, wires, foils, slabs, rods, discs and powder.
Chemical properties
grey metal ingot
Physical properties
Holmium is a crystal-like, solid rare-earth with a metallic luster. It is one of the more scarceelements of the lanthanide series. It is soft, like lead, and can be hammered and pounded intothin sheets.
Its melting point is 1,474°C, its boiling point is 2,700°C, and its density is 8.79g/cm3.
Isotopes
There are a total of 57 isotopes of holmium. Only one of these, Ho-165, is stable,and it is the only isotope found in the Earth’s crust. All the other 56 isotopes havehalf-lives of a few milliseconds to 1.20×10+3 years, the half-life of Ho-166.
Origin of Name
Derived from the Latin word for the ancient city named Holmia (present- day Stockholm, located in Sweden).
Occurrence
Holmium is the 12th most abundant of the rare-earths found in the Earth’s crust. Althoughit is the 50th most abundant element on Earth, it is one of the least abundant lanthanide metals.It is found in gadolinite and the monazite sands of South Africa and Australia and in thebeach sands of Florida and the Carolinas in the United States. Monazite sand contains about a50% mixture of the rare-earths, but only 0.05% by weight is holmium. Today, small quantitiesof holmium are produced by the ion-exchange process.
History
The spectral absorption bands of holmium were noticed in 1878 by the Swiss chemists Delafontaine and Soret, who announced the existence of an “Element X.” Cleve, of Sweden, later independently discovered the element while working on erbia earth. Holmium is named after Cleve’s native city. Pure holmia, the yellow oxide, was prepared by Homberg in 1911. Holmium occurs in gadolinite, monazite, and in other rare-earth minerals. It is commercially obtained from monazite, occurring in that mineral to the extent of about 0.05%. It has been isolated by the reduction of its anhydrous chloride or fluoride with calcium metal. Pure holmium has a metallicto bright silver luster. It is relatively soft and malleable, and is stable in dry air at room temperature, but rapidly oxidizes in moist air and at elevated temperatures. Holmium has unusual magnetic properties. Few uses have yet been found for the element. The element, as with other rare earths, seems to have a low acute toxic rating. Natural holmium consists of one isotope 165Ho, which is not radioactive. Holmium has 49 other isotopes known, all of which are radioactive. The price of 99.9% holmium metal is about $20/g.
Characteristics
Although stable at room temperatures, holmium will corrode at higher temperatures andhumidity. Its oxide coating is a yellowish film that reacts slowly with water and dissolves inweak acids. Holmium has one of the highest magnetic properties of any substance, but it haslittle commercial use.
The Uses of HOLMIUM
Holmium has just a few commercial uses, but it could be developed to produce items requiringstrong permanent magnets. It is used for filaments in vacuum tubes and in electrochemistry.It is also used to help identify the atomic weights of elements by spectroscopy, which identifiesthe unique lines produced by each element when viewed through a spectroscope. It also haslimited use as neutron absorber in nuclear reactors and as coloring agent for glass.
The Uses of HOLMIUM
Getter in vacuum tubes, research in electrochemistry, spectroscopy.
The Uses of HOLMIUM
Used in Physical Vapor Deposition (PVD) processes, including thermal and electron-beam (e-beam) evaporation, for the preparation of thin films.
Definition
Metallic element of atomic number 67, group IIIB of the periodic table, one of the rare-earth elements of the yttrium subgroup, aw 164.9303, valence of 3, no stable isotopes.
Definition
A soft malleable silvery element of the lanthanoid series of metals. It occurs in association with other lanthanoids. It has few applications. Symbol: Ho; m.p. 1474°C; b.p. 2695°C; r.d. 8.795 (25°C); p.n. 67; r.a.m. 164.93032.
Definition
holmium: Symbol Ho. A soft silverymetallic element belonging to thelanthanoids; a.n. 67; r.a.m. 164.93;r.d. 8.795 (20°C); m.p. 1474°C; b.p.2695°C. It occurs in apatite, xenotime,and some other rare-earth minerals.There is one natural isotope,holmium–165; eighteen artificial isotopeshave been produced. There areno uses for the element, which wasdiscovered by Per Cleve (1840–1905)and J. L. Soret in 1879.
Production Methods
Holmium is obtained from monazite, bastnasite and other rare-earth minerals as a by-product during recovery of dysprosium, thulium and other rareearth metals. The recovery steps in production of all lanthanide elements arevery similar. These involve breaking up ores by treatment with hot concentrated sulfuric acid or by caustic fusion; separation of rare-earths by ionexchange processes; conversion to halide salts; and reduction of the halide(s)to metal (See Dysprosium, Gadolinium and Erbium).
Bulk holmium metal is prepared by reduction of holmium chloride or fluo-ride by sodium, calcium, or magnesium in a tantalum crucible under argonatmosphere:
2HoF3 + 3Ca → 3CaF2 + 2Ho
Pure holmium metal is obtained by distillation of crude metal at 1,500°C.
General Description
Holmium Wavelength Calibration Standard is a high-quality secondary reference standard offered as a suitable alternative to NBS Holmium Oxide Solution (No. 2034). It is used to establish the accuracy of conventional spectrophotometers in the spectral range 240 to 650 nm. The product consists of Holmium perchlorate (15% w/v) in 10% perchloric acid.
Hazard
Because holmium is produced in such very small amounts, it is not a potential hazard tothe general public. However, professional chemists take the same precautions as they do withother rare-earths.
Properties of HOLMIUM
| Melting point: | 1474 °C |
| Boiling point: | 2695 °C |
| Density | 8.8 g/mL at 25 °C (lit.) |
| solubility | soluble in dilute acid solutions |
| form | ingot |
| color | Silver-gray |
| Specific Gravity | 8.795 |
| Water Solubility | reacts slowly with H2O, soluble dilute acids [HAW93] |
| Sensitive | Air & Moisture Sensitive |
| Merck | 13,4747 |
| Exposure limits | ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| CAS DataBase Reference | 7440-60-0(CAS DataBase Reference) |
| EPA Substance Registry System | Holmium (7440-60-0) |
Safety information for HOLMIUM
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H228:Flammable solids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P370+P378:In case of fire: Use … for extinction. |
Computed Descriptors for HOLMIUM
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 Holmium pieces, Distilled dendritic CAS 7440-60-0View Details
Holmium pieces, Distilled dendritic CAS 7440-60-0View Details
7440-60-0 -
 Holmium pieces, Distilled dendritic CAS 7440-60-0View Details
Holmium pieces, Distilled dendritic CAS 7440-60-0View Details
7440-60-0 -
 Holmium pieces CAS 7440-60-0View Details
Holmium pieces CAS 7440-60-0View Details
7440-60-0 -
 Holmium pieces CAS 7440-60-0View Details
Holmium pieces CAS 7440-60-0View Details
7440-60-0 -
 Holmium CAS 7440-60-0View Details
Holmium CAS 7440-60-0View Details
7440-60-0 -
 Holmium CAS 7440-60-0View Details
Holmium CAS 7440-60-0View Details
7440-60-0 -
 Holmium rod, ≈6.35mm (0.25 in.) dia. CAS 7440-60-0View Details
Holmium rod, ≈6.35mm (0.25 in.) dia. CAS 7440-60-0View Details
7440-60-0 -
 Holmium wavelength calibration standard CASView Details
Holmium wavelength calibration standard CASView Details