HMN-214
- CAS NO.:173529-46-9
- Empirical Formula: C22H20N2O5S
- Molecular Weight: 424.47
- MDL number: MFCD12756255
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
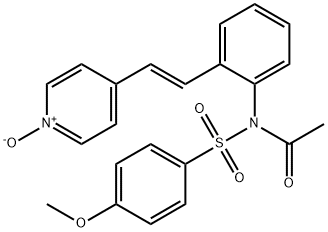
What is HMN-214?
Description
HMN-214 is an orally bioavailable prodrug form of HMN-176, an indirect inhibitor of polo-like kinase (PLK) activity that inhibits proliferation of a variety of cancer cells. HMN-214 decreases the expression of multidrug resistance gene 1 (MDR1) in AB-A.1 cells and in tumors isolated from mice bearing multidrug-resistant KB-A1 xenografts. HMN-214 (20 mg/kg per day) reduces tumor volume in PC3, WiDr, and A549 mouse xenograft models. It does not decrease nerve conduction velocity or compound action potential amplitude in rabbit sciatic nerves in vivo when administered at a concentration of 30 mg/kg per day.
The Uses of HMN-214
HMN 214 is a novel oral stilbene derivative with polo-like kinase-1-interacting properties, in patients with advanced solid tumors. It is a prodrug of HMN 176, a stilbene derivative that interferes with the subcellular spatial location of polo-like kinase-1 (PLK1).
What are the applications of Application
HMN-214 is a potent inhibitor of Polo-like kinase and a broad-spectrum anti-tumor agent
Definition
ChEBI: N-(4-methoxyphenyl)sulfonyl-N-[2-[2-(1-oxido-4-pyridin-1-iumyl)ethenyl]phenyl]acetamide is a sulfonamide.
in vitro
hmn-176 showed potent cytotoxicity, with a mean ic50 of 118 nm. the cytotoxic effecacy of hnm-176 was superior to that of adm, vp-16 and cddp, but inferior to that of taxol and vcr. hmn-176 showed less vaiance in log(ic50) than did the reference agents. in addition, judging by its low resistance indices, hmn-176 was more cytotoxic toward the drug-resistant phenotypes of tumor cells than were the other agents tested [1].
in vivo
pk studies showed that hnm-214 was an acceptable oral prodrug of hmn-176. in the in vivo analysis of the schedule-dependency of hmn-214, the repeated administration for over 5 days elicited potent antitumor activity, as expected from the exposure-dependency of the cytotoxicity of hmn-176 and from the cytometric studies. the antitumor activity of hmn-214 against human tumor xenografts was equal or superior to that of clinically avaiable agents, including cis-platinum, adriamycin, vincristine and uft without severe toxicity such as neurotoxicity [1].
References
[1] takagi m, honmura t, watanabe s, yamaguchi r, nogawa m, nishimura i, katoh f, matsuda m, hidaka h. in vivo antitumor activity of a novel sulfonamide, hmn-214, against human tumor xenografts in mice and the spectrum of cytotoxicity of its active metabolite, hmn-176. invest new drugs. 2003 nov;21(4):387-99.
[2] garland ll, taylor c, pilkington dl, cohen jl, von hoff dd. a phase i pharmacokinetic study of hmn-214, a novel oral stilbene derivative with polo-like kinase-1-interacting properties, in patients with advanced solid tumors. clin cancer res. 2006 sep 1;12(17):5182-9.
Properties of HMN-214
| Boiling point: | 663.1±65.0 °C(Predicted) |
| Density | 1.24 |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | ≥21.2 mg/mL in DMSO; insoluble in H2O; insoluble in EtOH |
| form | solid |
| pka | 0.86±0.10(Predicted) |
| color | White to off-white |
Safety information for HMN-214
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for HMN-214
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![4H-PYRIDO[1,2-A]PYRIMIDIN-4-ONE, 7-METHYL-2-(4-MORPHOLINYL)-9-[1-(PHENYLAMINO)ETHYL]-](https://img.chemicalbook.in/CAS/GIF/663619-89-4.gif)
![(2S)-1-[3-Ethyl-7-[[(1-oxido-3-pyridinyl)methyl]amino]pyrazolo[1,5-a]pyrimidin-5-yl]-2-piperidineethanol](https://img.chemicalbook.in/CAS/GIF/779353-01-4.gif)

![N-[2-(1H-Indol-3-yl)ethyl]-N'-(4-pyridinyl)-1,4-benzenediamine](https://img.chemicalbook.in/CAS2/GIF/881202-45-5.gif)
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1