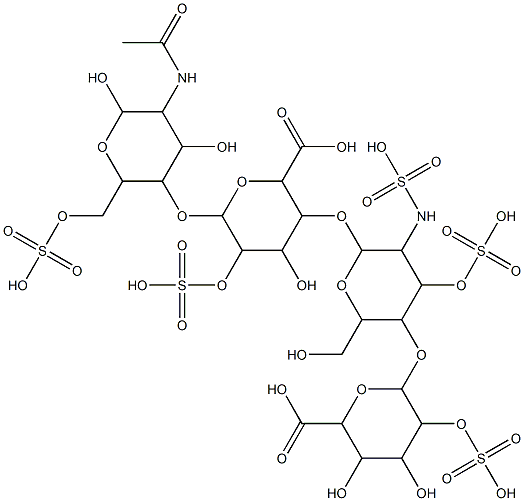
HEPARIN
- CAS NO.:91449-79-5
- Empirical Formula: C26H42N2O37S5
- Molecular Weight: 1134.92788
- MDL number: MFCD00131311
- Update Date: 2024-11-20 17:10:30
What is HEPARIN?
Absorption
Hemiparin sodium is rapidly absorbed following its subcutaneous dose of injection, and the bioavailability is estimated to be 96% .
Toxicity
Bemiparin, like other drugs in its class, may suppress adrenal secretion of aldosterone, leading to elevated potassium (hyperkalemia). This may occur more frequently in patients with conditions such as diabetes mellitus, chronic renal failure, metabolic acidosis, an increased plasma potassium, and those ingesting potassium sparing drugs. There is a linear relationship between duration of therapy and adverse effects, but this is usually reversible with cessation of treatment. Serum electrolytes should be measured in at-risk patients before starting bemiparin, and these patients should be monitored regularly thereafter particularly if treatment is prolonged beyond 1 week.
In rare cases, mild transient thrombocytopenia (HIT type I) at the beginning of therapy with heparin with platelet counts between 100,000/mm3 and 150,000/mm3 due to temporary platelet activation has been noted in clinical studies.
On rare occasions, antibody-mediated severe thrombocytopenia (HIT type II) with platelet counts clearly below
100,000/mm3 has been observed (see section 4.8). This effect usually occurs within 5-21 days after the initiation of treatment, although in patients with a history of heparin-induced thrombocytopenia this may occur more rapidly.
Platelet count studies are recommended before the administration of bemiparin, on the first day of therapy and then every 3-4 days, in addition to repeating platelet studies at the end of therapy. Treatment must be discontinued immediately and an alternate therapy initiated if significant reductions in platelet counts are observed ( 30% decrease and above) .
As with other heparin products, cases of cutaneous necrosis, often preceded by purpura or painful erythematous, ecchymose-like lesions have been reported with bemiparin. In these cases, treatment should cease immediately .
Overdosage after subcutaneous or other routes of administration of bemiparin may lead to hemorrhagic complications. Neutralization can be obtained by slow intravenous of a suitable dose of the antidote protamine sulphate .
Description
Enoxaparin is a low molecular weight heparin fraction obtained by controlled depolymerization of pig heparin. As an anticoagulant it is reportedly useful in the treatment of venous thromboembolism, and the prevention of coagulation in the extracorporeal circulation during hemodialysis.
Originator
Pharmuka (France)
Indications
Bemiparin is indicated in the following cases: To prevent blood clots in the veins after general abdominal surgery in patients with a moderate risk of venous thromboembolism; in the prevention of the thromboembolic disease in non-surgical patients; prevention of clotting in the extracorporeal circuit during hemodialysis; to prevent blood clots in the veins after a major orthopedic surgery in patients with high risk of venous thromboembolism; secondary prevention of venous thromboembolism; recurrence in patients with deep vein thrombosis; transient prevention and treatment of deep vein thrombosis (DVT) .
Background
Bemiparin is an antithrombotic and belongs to the group of drugs known as the low molecular weight heparins (LMWH). Like semuloparin, bemiparin is classified as an ultra-LMH because of its low mean molecular mass of 3600 daltons, which is a unique property of this class . These heparins have lower anti-thrombin activity than the traditional low molecular weight heparins and act mainly on factor-Xa, reducing the risk of bleeding due to selectivity for this specific clotting factor. Interestingly, current research is underway for the potential benefit of bemiparin in the treatment of tumors and diabetic foot ulcers .
brand name
Lovenox
Pharmacokinetics
Bemiparin is an anticoagulant classified under the broad category of low molecular weight heparins. In humans, bemiparin has been proven to possess antithrombotic activity and, at therapeutic doses, does not significantly prolong global clotting laboratory tests .
Metabolism
In a study of healthy volunteers, bemiparin 3500 IU achieved more anti-Xa activity than enoxaparin 4000 IU, measured by the area under the curve. The peak of anti-Xa activity was reached at 3h post-administration, and there were anti-Xa measurable levels up to 16 h after subcutaneous injection .
Safety information for HEPARIN
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran





You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1