HARMANE
Synonym(s):1-Methyl-9H-pyrido[3,4-b]indole;2-Methyl-β-carboline;Aribine
- CAS NO.:486-84-0
- Empirical Formula: C12H10N2
- Molecular Weight: 182.22
- MDL number: MFCD00004957
- EINECS: 207-642-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
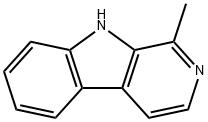
What is HARMANE?
Description
Chrysin (on the left) and harman (right) are organic compounds with distinctly different structures, but both are found in the passionflower species Passiflora caerulea and P. incarnata.
Chrysin, also called 5,7-dihydroxyflavone, was first isolated from the wood of pine trees (Pinus spp.) in 1949 by G?sta Linstedt at the KTH Royal Institute of Technology (Stockholm). Harman (or harmane), a pyridoindole derivative, was discovered much earlier (1861) in the bark of P. incarnata and other trees by German researcher R. Rieth.
What do chrysin and harman have in common besides occurrence in trees, specifically P. incarnata? More than 500 passionflower species have been used as traditional folk remedies for anxiety and other medical conditions almost everywhere that they grow on Earth. For at least 20 years, drug researchers have sought to elucidate mechanisms by which passionflower biochemicals provide relief.
In a key 2001 study, P. incarnata extract was compared with oxazepam, an early benzodiazepine anxiolytic drug, for efficacy against generalized anxiety disorder (GAD). Shaheen Akhondzadeh and colleagues at the Tehran University of Medical Sciences and the Institute of Medicinal Plants (both in Tehran, Iran) treated 36 patients diagnosed with GAD with P. incarnata extract, oxazepam, or placebo in a 4-week trial.
The extract and the drug gave equally positive results. Oxazepam acted more rapidly, but it also impaired the subjects’ job performance whereas the extract did not. In the time since this report was issued, however, there is no record of US Food and Drug Administration filings for chrysin, harman, or passionflower extracts.
This discussion of anxiety remedies reminds us that today is the beginning of Mental Health Awareness Week.
Chemical properties
Off-White Solid
The Uses of HARMANE
Harman alkaloid like harmane, harmine, harmalol, harmaline obtained from Banisteriopsis caapi L. showed cytotoxicity, antimicrobial activity against Staphylococcus aureus, Escherichia coli, Proteus vulgaris and Candida albicans.
The Uses of HARMANE
- Harmane was used in trace level determination of harmane by planar chromatography coupled with (tandem) mass spectrometry.
- It was used to study interactions of norharman and harman with DNA.
- It may be used as matrix for analysis of cyclodextrins and for sulfated oligosaccharides in combination with DHB as co-matrix.
What are the applications of Application
Harmane is an endogenous MAO-A and -B inhibitor (Order -CW for certified weight, analytical data, etc.)
Definition
ChEBI: An indole alkaloid fundamental parent with a structure of 9H-beta-carboline carrying a methyl substituent at C-1. It has been isolated from the bark of Sickingia rubra, Symplocus racemosa, P ssiflora incarnata, Peganum harmala, Banisteriopsis caapi and Tribulus terrestris, as well as from tobacco smoke. It is a specific, reversible inhibitor of monoamine oxidase A.
Synthesis Reference(s)
The Journal of Organic Chemistry, 37, p. 1429, 1972 DOI: 10.1021/jo00974a030
Tetrahedron, 49, p. 3325, 1993 DOI: 10.1016/S0040-4020(01)90161-9
General Description
- Harmane is a potent tremor-producing β-carboline alkaloid and neurotoxin.
- It is major representative of heterocyclic aromatic amines, a group of mutagenic and carcinogenic substances which are formed in meat from the precursors creatine, creatinine, amino acids and sugars during the heating at high temperatures.
- Blood harmane concentration is elevated in essential tremor, late-life neurological disease.
Biological Activity
Proposed as the endogenous ligand for imidazoline binding sites. Binds to I 1 -sites in rat kidney with an IC 50 of 31 nM, and I 2 -sites with a K i of 49 nM. In vivo, produces a dose-dependent hypotension that is reversed by efaroxan (2-(2-Ethyl-2,3-dihydro-2-benzofuranyl)-4,5-dihydro-1H-imidazole hydrochloride ). Also a potent inhibitor of monoamine oxidases A and B (I 50 values are 0.5 and 5 μ M respectively).
Biochem/physiol Actions
I1 imidazoline binding site agonist.
storage
Room temperature
Properties of HARMANE
| Melting point: | 235-238 °C(lit.) |
| Boiling point: | 305.62°C (rough estimate) |
| Density | 1.1485 (rough estimate) |
| refractive index | 1.6266 (estimate) |
| storage temp. | Store at RT |
| solubility | methanol: soluble50mg/ml |
| form | Solid |
| appearance | white, light beige, or light yellow crystals or powder |
| pka | 7.37, 14.6(at 25℃) |
| color | White to Dark Brown |
| Water Solubility | 1523g/L(20 ºC) |
| Merck | 13,4630 |
| BRN | 143898 |
| CAS DataBase Reference | 486-84-0(CAS DataBase Reference) |
Safety information for HARMANE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for HARMANE
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran






You may like
-
 Harmane 95% CAS 486-84-0View Details
Harmane 95% CAS 486-84-0View Details
486-84-0 -
 Harmane CAS 486-84-0View Details
Harmane CAS 486-84-0View Details
486-84-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8