HALOFANTRINE
- CAS NO.:69756-53-2
- Empirical Formula: C26H30Cl2F3NO
- Molecular Weight: 500.42
- MDL number: MFCD00866195
- EINECS: 274-104-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
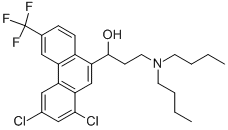
What is HALOFANTRINE?
Toxicity
Side effects incldue coughing noisy, rattling, troubled breathing, loss of appetite, aches and pain in joints, indigestion,and skin itching or rash.
Description
Halofantrine is an orally-active blood schizonticide reportedly highly effective in the treatment of fulcipurum malaria and other types of parasitemia. Cure rate is claimed to be over 95%.
Originator
Smith mine & French (USA)
Background
Halofantrine is an antimalarial. It belongs to the phenanthrene class of compounds that includes quinine and lumefantrine. It appears to inhibit polymerisation of heme molecules (by the parasite enzyme "heme polymerase"), resulting in the parasite being poisoned by its own waste. Halofantrine has been shown to preferentially block open and inactivated HERG channels leading to some degree of cardiotoxicity.
Indications
For treatment of Severe malaria
Definition
ChEBI: 3-(dibutylamino)-1-[1,3-dichloro-6-(trifluoromethyl)-9-phenanthrenyl]-1-propanol is a member of phenanthrenes.
brand name
Halfan
World Health Organization (WHO)
Halofantrine is an antimalarial introduced to medicine in 1982. It should be reserved for use in areas where multiple drug-resistant falciparum malaria is prevalent. Cases of serious cardiotoxicity have been reported.
Antimicrobial activity
It inhibits erythrocytic stages of chloroquine-sensitive and chloroquine-resistant P. falciparum and other Plasmodium spp. in vitro at concentrations of 0.4–4.0 mg/L. It is more active than mefloquine and the combination of proguanil and atovaquone against P. falciparum, but less effective than mefloquine or chloroquine against P. vivax.
Acquired resistance
Resistance in P. falciparum has been reported in Central and West Africa, where it has been used widely. Cross-resistance with mefloquine has been reported in Thailand, where it has not been used.
General Description
Structurally, halofantrine differs from allother antimalarial drugs. It is a good example of drug designthat incorporates bioisosteric principles as evidenced by thetrifluromethyl moiety. Halofantrine is a schizonticide and has no affect on the sporozoite, gametocyte,or hepatic stages. Both the parent compound and Ndesbutylmetabolite are equally active in vitro. Halfantrine’sspecific mechanism of action against the parasite is notknown. There is contradictory evidence that its mechanismranges from requiring heme to disrupting the mitochondria.There is a prominent warning that halfantrine can affectnerve conduction in cardiac tissue.
Pharmaceutical Applications
A phenanthrene methanol, formulated as the hydrochloride for oral administration. Parenteral formulations are not available. The enantiomers have equivalent activity in vitro. Aqueous solubility is extremely low.
Pharmacokinetics
Halofantrine is a synthetic antimalarial which acts as a blood schizonticide. It is effective against multi drug resistant (including mefloquine resistant) P. falciparum malaria.
Pharmacokinetics
Absorption shows high intra- and inter-subject variability and depends on co-administration with fats. Bioavailability is increased more than six-fold after a fatty meal or by lipidbased formulations. Bioavailability is significantly lower in patients with malaria than in healthy individuals. Peak plasma levels are variable and occur 3–6 h after administration. Unlike many other antimalarials, halofantrine is not concentrated by infected or uninfected erythrocytes. Distribution to lipoproteins is stereo-selective. About 20–30% of the dose is metabolized to an N-desbutyl derivative by cytochrome P450 (CYP) 3A4 and 3A5. The elimination half-life of the parent drug is generally 1–2 days and that of the metabolite 3 days. Little unchanged drug is excreted in urine.
Clinical Use
Treatment of multidrug-resistant falciparum malaria
Its use has been questioned due to the existence of safer
alternatives.
Side Effects
Abdominal pain, diarrhea and pruritus are the most frequent. High doses (24 mg/kg) induce prolongation of the PR and QTc intervals; this is not stereo-selective. There are individual reports of fatal cardiac arrest and torsade de pointes. To reduce the risk of cardiac toxicity it should be taken on an empty stomach. It should not be administered with other antimalarials that have the potency to induce cardiac arrhythmias (mefloquine, chloroquine, quinine). Halofantrine has also been associated with intravascular hemolysis.
Metabolism
Hepatic
Metabolism
Halofantrine is considered to be an alternative drug for treatment of both chloroquine-sensitive and chloroquine-resistant Plasmodium falci parum malaria, but its efficacy in mefloquine-resistant malaria may be questionable. The drug is metabolized via N-dealkylation to desbutylhalofantrine by CYP3A4. The metabolite appears to be several-fold more active than the administered drug.
Properties of HALOFANTRINE
| Density | 1.244±0.06 g/cm3 (20 ºC 760 Torr) |
| storage temp. | 2-8°C |
| Water Solubility | 0.59mg/L(37 ºC) |
Safety information for HALOFANTRINE
Computed Descriptors for HALOFANTRINE
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
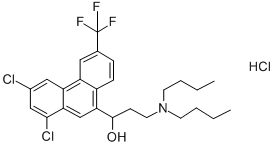
 Halofantrine HCl 98% (HPLC) CAS 69756-53-2View Details
Halofantrine HCl 98% (HPLC) CAS 69756-53-2View Details
69756-53-2 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8