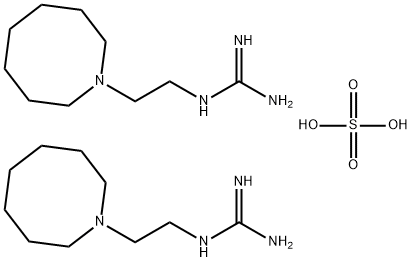
GUANETHIDINE SULFATE
Synonym(s):1-(2-Guanidinoethyl)octahydroazocine;Guanethidine monosulfate (1:1)
- CAS NO.:645-43-2
- Empirical Formula: C10H24N4O4S
- Molecular Weight: 296.39
- MDL number: MFCD00035403
- EINECS: 211-442-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-25 20:05:57
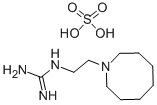
What is GUANETHIDINE SULFATE?
Description
Guanethidine is an antihypertensive compound that competes with norepinephrine for transport into presynaptic terminals of adrenergic neurons by the norepinephrine transporter. Once guanethidine has entered the nerve, it becomes concentrated in synaptic vesicles, depleting endogenous norepinephrine, and thus, reducing the release of norepinephrine in response to action potentials. Guanethidine’s actions are restricted to peripheral nerve terminals because its basic guanidine group does not allow passage through the blood brain barrier. Its use has been explored in the relief of chronic pain caused by complex regional pain syndrome.
Chemical properties
GUANETHIDINE SULFATE is colourless, crystalline powder
Originator
Ismelin,Ciba,US,1960
The Uses of GUANETHIDINE SULFATE
Antihypertensive.
The Uses of GUANETHIDINE SULFATE
Guanethidine Monosulfate acts as an antihypertensive; antiglaucoma
What are the applications of Application
Guanethidine sulfate is an antihypertensive and antiglaucoma compound, competitive with norepinephrine in adrenergic neuron transport.
Definition
ChEBI: A organic sulfate salt obtained from guanethidine and sulfuric acid in a 1:1 ratio.
Manufacturing Process
13.6 grams of chloroacetyl guanide is added while stirring to a solution of 22.6 grams of heptamethylene imine in 200 ml of benzene. After warming for 1 hour, and then cooling, the solution is filtered and the filtrate concentrated under reduced pressure. The residue, containing the 2-(1-N,N-heptamethylene-imino)-aceticacid guanide, is suspended in tetrahydrofuran and added to a refluxing solution of 6 grams of lithium aluminum hydride in tetrahydrofuran. After completion of the reaction, the excess of lithium aluminum hydride is decomposed by adding water, then aqueous sodium hydroxide. The solid material is filtered off, the filtrate is acidified with sulfuric acid and the 2-(1-N,N-heptamethylene-imino)-ethyl-guanidine sulfate can be recovered and recrystallized from aqueous ethanol, MP 276° to 281°C (with decomposition).
brand name
Ismelin (Novartis).
Therapeutic Function
Antihypertensive
General Description
Guanethidinemonosulfate, [2-(hexahydro-1 (2H)-azocinyl)ethyl]guanidinesulfate (Ismelin sulfate), is a white, crystalline materialthat is very soluble in water. It was one of a series ofguanidine compounds prepared in the search for potent antitrypanosomalagents. There is an absence of CNS effects,such as depression, because the drug is highly polar anddoes not easily cross the blood-brain barrier. Guanethidinemonosulfate produces a gradual, prolonged fall in bloodpressure. Usually, 2 to 7 days of therapy are required beforethe peak effect is reached, and usually, this peak effectis maintained for 3 or 4 days. Then, if the drug is discontinued,the blood pressure returns to pretreatment levelsover a period of 1 to 3 weeks. Because of this slow onsetand prolonged duration of action, only a single daily doseis needed.
Mechanism of action
Guanethidine is an adrenergic neuronal blocking agent that produces a selective block of peripheral sympathetic pathways by replacing and depleting norepinephrine stores from adrenergic nerve endings, but not from the adrenal medulla. It prevents the release of norepinephrine from adrenergic nerve endings in response to sympathetic nerve stimulation. The chronic administration of guanethidine results in an increased sensitivity of these effector cells to catecholamines. Following the oral administration of usual doses of guanethidine, depletion of the catecholamine stores from adrenergic nerve endings occurs at a very slow rate, producing a more gradual and prolonged fall in systolic blood pressure than in diastolic pressure. Associated with the decrease in blood pressure is an increase in sodium and water retention and expansion of plasma volume (edema). If a diuretic is not administered concurrently with guanethidine, tolerance to the antihypertensive effect of the guanethidine during prolonged therapy can result.
Pharmacokinetics
Guanethidine is incompletely absorbed from the GI tract and is metabolized in the liver to several metabolites, including guanethidine N-oxide (from flavin mononucleotide). These metabolites of guanethidine are excreted in the urine and have less than 10% of its hypotensive activity. The amount of drug that reaches the systemic circulation after oral administration is highly variable from patient to patient and may range from 3 to 50% of a dose. Guanethidine accumulates in the neurons with an elimination half-life of 5 days.
Clinical Use
Guanethidine monosulfate is metabolized by microsomalenzymes to 2-(6-carboxyhexylamino)ethylguanidine andguanethidine N-oxide . Both metabolites havevery weak antihypertensive properties. Guanethidine monosulfateis taken up by the amine pump located on theneuronal membrane and retained in the nerve, displacingnorepinephrine from its storage sites in the neuronal granules.The displaced norepinephrine is metabolized to homovanillicacid by mitochondrial MAO, depleting the nerveending of the neurotransmitter. The usefulness of guanethidinemonosulfate also resides in the fact that once it is takenup by the nerve, it produces a sympathetic blockade by inhibitingrelease of nonepinephrine that would occur on neuronalmembrane response to stimulation29 by the nerveaction potential. Guanethidine monosulfate stored in thegranules is released by the nerve action potential but hasvery low intrinsic activity for the adrenergic receptors on thepostjunctional membrane. Moderate doses for a prolongedperiod or large doses may produce undesirable side effectsby causing neuromuscular blockade and adrenergic nerveconduction blockade.
Side Effects
Adverse effects of guanethidine frequently are dose related, including dizziness, weakness, lassitude, and syncope resulting from postural or postexercise hypotension. A hot environment (i.e., a hot bath) may aggravate postural hypotension. Patients should be warned about possible orthostatic hypotension and about the effect of rapid postural changes on blood pressure (e.g., arising in the morning) that may cause fainting, especially during the initial period of dosage adjustment. Sodium retention (edema) usually is controlled by the coadministration of a diuretic.
Drug interactions
Diuretics and other hypotensive drugs can potentiate the hypotensive effects of guanethidine. Reportedly, MAO
inhibitors antagonize the hypotensive effect of guanethidine. Oral sympathomimetic, nasal decongestants, and other
vasopressor agents should be used cautiously in patients receiving guanethidine, because guanethidine may
potentiate their pressor effects. The mydriatic response to ophthalmic administration of phenylephrine is markedly
increased in patients receiving guanethidine either ophthalmically or orally.
Tricyclic antidepressants and some phenothiazines block the uptake of guanethidine into adrenergic neurons and,
thus, prevent the hypotensive activity of guanethidine. Orthostatic hypotension may be increased by concomitant
administration of alcohol with guanethidine, and patients receiving guanethidine should be cautioned to limit alcohol
intake.
Properties of GUANETHIDINE SULFATE
| Melting point: | 276-281 °C |
| storage temp. | 2-8°C |
| solubility | Freely soluble in water, practically insoluble in ethanol (96 per cent) |
| form | Solid |
| color | White to Off-White |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 645-43-2(CAS DataBase Reference) |
Safety information for GUANETHIDINE SULFATE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P501:Dispose of contents/container to..… |
Computed Descriptors for GUANETHIDINE SULFATE
| InChIKey | YUFWAVFNITUSHI-UHFFFAOYSA-N |
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

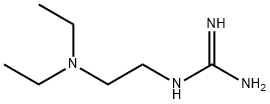
![[2-(Diethylamino)ethyl]guanidinium sulfate](https://img.chemicalbook.in/CAS/GIF/3272-63-7.gif)
You may like
-
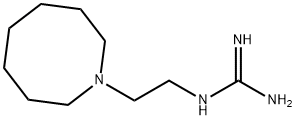
 Guanethidine monosulfate CAS 645-43-2View Details
Guanethidine monosulfate CAS 645-43-2View Details
645-43-2 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4