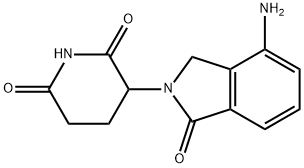
GSK1120212 (DMSO solvate)
- CAS NO.:1187431-43-1
- Empirical Formula: C28H29FIN5O5S
- Molecular Weight: 693.53
- MDL number: MFCD21609250
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-17 19:25:39
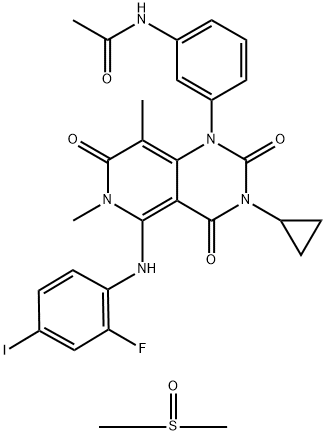
What is GSK1120212 (DMSO solvate)?
Description
In May 2013, the US FDA approved trametinib (also referred to as GSK1120212 and JTP-74057), for the treatment of patients with unresectable or metastatic melanoma with BRAFV600e or BRAFV600K mutations as detected by an FDA-approved test. Extensive lead optimization led to the identification of trametinib which is a potent ATP noncompetitive inhibitor of MEK1 and MEK2 (IC50 =0.7 and 0.9 nM, respectively, with initially unphosphorylated MEK). It also showed inhibitory activity in ACHN and HT-29 cancer cell lines (IC50s of 9.8 and 0.57 nM, respectively). Consistent with its in vitro activity, trametinib showed significant antitumor activity in a KRASG12S A549 tumor xenograft model where near to complete tumor growth inhibition (TGI) was observed at 5.0 and 2.5 mg/kg (92% and 87% TGI, respectively). Broad antitumor activity was seen in other xenograft models as well. A synthetic route to trametinib that employs a base catalyzed rearrangement of a pyrido[2,3-d]pyrimidine core to pyrido[4,3-d]pyrimidine, as a key step has been reported.
Originator
Japan Tobacco (Japan)
The Uses of GSK1120212 (DMSO solvate)
Trametinib (DMSO Solvate) is a highly potent and selective MEK inhibitor with significant antitumor activity.
Definition
ChEBI: An addition compound obtained by combining equimolar amounts of trametinib and dimethyl sulfoxide. Used for the treatment of patients with unresectable or metastatic melanoma with BRAF V600E or V600K mutations, and who have not received prior BRAF inhibito treatment.
brand name
Mekinist
Synthesis
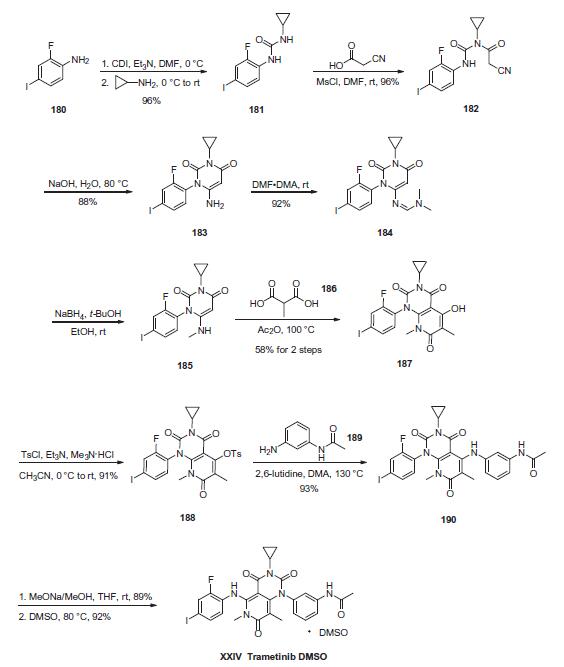
Commercial 2-fluoro-4-iodoaniline (180) was sequentially subjected to CDI and cyclopropylamine to generate urea 181 in 96% yield. This was followed by coupling with cyanoacetic acid in the presence of mesyl chloride and DMF to furnish imide 182 in 96% yield. Under basic conditions, imide 182 underwent an intramolecular cyclization reaction to produce pyrimidine-2,4- dione 183 in 88% yield. Next, condensation with DMF¨CDMA generated formamidine 184 in 92% yield, and this was followed by NaBH4-mediated reduction and subsequent annulation with 2- methyl-malonic acid (186) to arrive at trione 187 in 58% from 184. Trione 187 was then treated with p-toluenesulfonyl chloride in Et3N, and the resulting tosylate was exposed to 30-aminoacetanilide (189) in the presence of 2,6-lutidine and DMA, inducing an addition-elimination reaction to give pyrido[2,3-d]pyrimidine 190 in 93% yield. The rearrangement of pyrido[2,3-d]pyrimidine 190 with sodium methoxide in THF/MeOH gave pyrido[4,3- d]pyrimidine (trametinib) in 89% yield. This was then complexed with a single equivalent of DMSO to produce trametinib DMSO (XXIV) in 92% yield.
Properties of GSK1120212 (DMSO solvate)
| storage temp. | Store at -20°C |
| solubility | insoluble in EtOH; insoluble in H2O; ≥11.2 mg/mL in DMSO with gentle warming and ultrasonic |
| form | solid |
| color | White to off-white |
Safety information for GSK1120212 (DMSO solvate)
Computed Descriptors for GSK1120212 (DMSO solvate)
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile Diclofenac Potassium Ornidazole IP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Aceclofenac IP/BP/EP Nimesulide BP SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION SODIUM METHYL PARABEN Methylcobalamin (vitamin B12) SODIUM VALPROATE LAMOTRIGINE RacecadotrilRelated products of tetrahydrofuran
You may like
-
![N-(3-(3-Cyclopropyl-5-((2-fluoro-4-iodophenyl)amino)-6,8-dimethyl-2,4,7-trioxo-3,4,6,7-tetrahydropyrido[4,3-d]pyrimidin-1(2h)-yl)phenyl)acetamide compound with (methylsulfinyl)methane (1:1) 95% CAS 1187431-43-1](https://img.chemicalbook.in//Content/image/CP5.jpg) N-(3-(3-Cyclopropyl-5-((2-fluoro-4-iodophenyl)amino)-6,8-dimethyl-2,4,7-trioxo-3,4,6,7-tetrahydropyrido[4,3-d]pyrimidin-1(2h)-yl)phenyl)acetamide compound with (methylsulfinyl)methane (1:1) 95% CAS 1187431-43-1View Details
N-(3-(3-Cyclopropyl-5-((2-fluoro-4-iodophenyl)amino)-6,8-dimethyl-2,4,7-trioxo-3,4,6,7-tetrahydropyrido[4,3-d]pyrimidin-1(2h)-yl)phenyl)acetamide compound with (methylsulfinyl)methane (1:1) 95% CAS 1187431-43-1View Details
1187431-43-1 -
 Trametinib Dimethyl Sulfoxide >95% CAS 1187431-43-1View Details
Trametinib Dimethyl Sulfoxide >95% CAS 1187431-43-1View Details
1187431-43-1 -
 89796-99-6 Aceclofenac IP/BP/EP 98%View Details
89796-99-6 Aceclofenac IP/BP/EP 98%View Details
89796-99-6 -
 61-68-7 98%View Details
61-68-7 98%View Details
61-68-7 -
 Diclofenac Sodium IP/BP/EP/USP 98%View Details
Diclofenac Sodium IP/BP/EP/USP 98%View Details
15307-79-6 -
 Ornidazole IP 16773-42-5 98%View Details
Ornidazole IP 16773-42-5 98%View Details
16773-42-5 -
 51803-78-2 Nimesulide BP 98%View Details
51803-78-2 Nimesulide BP 98%View Details
51803-78-2 -
 15307-81-0 98%View Details
15307-81-0 98%View Details
15307-81-0