Grubbs catalyst 2nd generation
Synonym(s):(1,3-Bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene)dichloro(phenylmethylene)(tricyclohexylphosphine)ruthenium;Benzylidene[1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene]dichloro(tricyclohexylphosphine)ruthenium;Dichloro[1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene](benzylidene)(tricyclohexylphosphine)ruthenium(II);Grubbs Catalyst 2nd Generation;Grubbs Catalyst M2a (C848)
- CAS NO.:246047-72-3
- Empirical Formula: C46H65Cl2N2PRu
- Molecular Weight: 848.98
- MDL number: MFCD03453237
- EINECS: 688-322-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-04-04 19:24:13
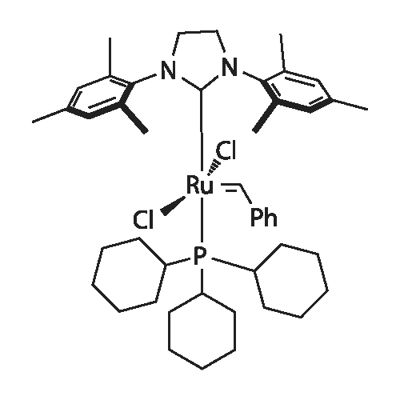
What is Grubbs catalyst 2nd generation?
Chemical properties
pink-brown to red-purple crystals or powder
Characteristics
Grubbs catalyst is a kind of coordination compound used in olefin metathesis reaction, and the ligand in it has a great influence on the catalytic performance and stability of the catalyst. Grubbs second-generation catalysts are more active than first-generation catalysts and have a wider substrate range, including sterically demanding or deactivated olefins such as 1,1-disubstituted olefins and α,β-non- Saturated carbonyl compounds.
The Uses of Grubbs catalyst 2nd generation
suzuki reaction
The Uses of Grubbs catalyst 2nd generation
Grubbs Catalyst? M204 can be used as a catalyst for ring-closing metathesis (RCM), cross-metathesis, and ring-opening metathesis polymerization (ROMP). It is also used to synthesize trisubstituted olefins with excellent functional group tolerance and selectivity via cross-metathesis and ring closing metathesis reactions.
It can also be used as a catalyst:
- To synthesize coumarins from phenolic compounds via RCM.
- To cleave secondary (E)-allyl vic-diols to aldehydes.
Definition
Adlhart and Chen found that ligand rotation is the difference between the activity of the first and second-generation Grubbs catalysts. This difference is brought about by a difference in the symmetry of the ligands. The phosphine ligand has a threefold symmetry, which causes a high barrier in the middle of the reaction coordinate, which is forced by the need for rotation of the phosphine. However, the barrier is absent for the second-generation Grubbs catalyst, with twofold symmetry of the NHC ligand. The rate-limiting step for second-generation catalysts is always phosphine dissociation[2].
Properties of Grubbs catalyst 2nd generation
| Melting point: | 143.5-148.5 °C(lit.) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Very Slightly, Heated) |
| form | Solid |
| color | Brown to Very Dark Brown to Very Dark Red |
| EPA Substance Registry System | Ruthenium, [1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene]dichloro(phenylmethylene)(tricyclohexylphosphine)-, (SP-5-41)- (246047-72-3) |
Safety information for Grubbs catalyst 2nd generation
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H228:Flammable solids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P370+P378:In case of fire: Use … for extinction. |
Computed Descriptors for Grubbs catalyst 2nd generation
| InChIKey | FCDPQMAOJARMTG-UHFFFAOYSA-L |
| SMILES | P(C1CCCCC1)(C1CCCCC1)(C1CCCCC1)[Ru+6](=[C-2]1N(C2C(=CC(C)=CC=2C)C)CCN1C1C(=CC(C)=CC=1C)C)([Cl-])([Cl-])=[C-2]C1C=CC=CC=1 |
Grubbs catalyst 2nd generation manufacturer
JSK Chemicals
New Products
4-Piperidinemethanol Ethyl 2,4-Dihydroxy-6-methylnicotinate Ethyl isonicotinate 3-pyridine methanol N-Methyl 4-chloro-pyridine-2-carboxamide 5,6 Dimethoxy-1-indanone 3-Iodophenylacetic acid 2-Hexyn-1-ol Dibenzo-18-crown-6 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Pyridineacetonitrile, α-hydroxy- 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole N Ethylmethylamine Ethyl Methanesulfonate N N' DimethylEthylenediamine Lead II Bromide Variamine Blue B Diazonium salt N N N'Trimethyl ethylenediamine Radiator Flux Zinc Chloride Solution (All Grades) Levetiracetam Fmoc-Gln-OH Boc Phenyl Alanine Alpha Lipoic Acid*Related products of tetrahydrofuran
bis(3-bromopyridine)ruthenium(II)](https://img.chemicalbook.in/CAS/GIF/900169-53-1.gif)
![DICHLORO-[1,3-BIS(DIISOPROPYLPHENYL)IMIDAZOLYLIDENE]-(3-CHLOROPYRIDYL)PALLADIUM(II)](https://img.chemicalbook.in/CAS/GIF/905459-27-0.gif)
You may like
-
 246047-72-3 98%View Details
246047-72-3 98%View Details
246047-72-3 -
 Grubbs Catalyst™ 2nd Generation 246047-72-3 99%View Details
Grubbs Catalyst™ 2nd Generation 246047-72-3 99%View Details
246047-72-3 -
 Grubbs catalyst, 2nd generation, 97% CAS 246047-72-3View Details
Grubbs catalyst, 2nd generation, 97% CAS 246047-72-3View Details
246047-72-3 -
 Grubbs Catalyst CAS 246047-72-3View Details
Grubbs Catalyst CAS 246047-72-3View Details
246047-72-3 -
 Grubbs Catalyst® M204 ChemBeads CAS 246047-72-3View Details
Grubbs Catalyst® M204 ChemBeads CAS 246047-72-3View Details
246047-72-3 -
 Grubbs Catalyst® M204 CAS 246047-72-3View Details
Grubbs Catalyst® M204 CAS 246047-72-3View Details
246047-72-3 -
 5162-90-3 2-Amino-3-(1,2-dihydro-2-oxoquinoline-4-yl)propanoic acid 97%View Details
5162-90-3 2-Amino-3-(1,2-dihydro-2-oxoquinoline-4-yl)propanoic acid 97%View Details
5162-90-3 -
 4-(4-Chlorobenzyl)-2-(1-methylazepan-4-yl)phthalazin-1(2H)-one hydrochloride 98 %View Details
4-(4-Chlorobenzyl)-2-(1-methylazepan-4-yl)phthalazin-1(2H)-one hydrochloride 98 %View Details
79307-93-0
