Granisetron
- CAS NO.:109889-09-0
- Empirical Formula: C18H24N4O
- Molecular Weight: 312.41
- MDL number: MFCD00865550
- EINECS: 686-533-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-02 08:55:04
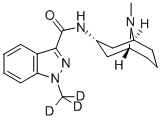
What is Granisetron?
Absorption
Absorption of is rapid and complete, though oral bioavailability is reduced to about 60% as a result of first pass metabolism.
Toxicity
LD50>2000 mg/kg (rat, oral)
The Uses of Granisetron
Granisetron is a selective 5HT-3 antagonist.
The Uses of Granisetron
Anti-emetic.
Indications
For the prevention of nausea and vomiting associated with initial and repeat courses of emetogenic cancer therapy (including high dose cisplatin), postoperation, and radiation (including total body irradiation and daily fractionated abdominal radiation).
Background
A serotonin receptor (5HT-3 selective) antagonist that has been used as an antiemetic and antinauseant for cancer chemotherapy patients.
What are the applications of Application
Granisetron is a selective 5HT-3 antagonist
Definition
ChEBI: A monocarboxylic acid amide resulting from the formal condensation of the carboxy group of 1-methyl-1H-indazole-3-carboxylic acid with the primary amino group of (3-endo)-9-methyl-9-azabicyclo[3.3.1]nonan-3-amine. A sele tive 5-HT3 receptor antagonist, it is used (generally as the monohydrochloride salt) to manage nausea and vomiting caused by cancer chemotherapy and radiotherapy, and to prevent and treat postoperative nausea and vomiting.
Indications
granisetron (Kytril) is potent antagonists of 5-HT3 receptors,which is found peripherally on vagal nerve terminals and centrally in the CTZ. During chemotherapy that induces vomiting, mucosal enterochromaffin cells in the GI tract release serotonin, which stimulates 5-HT3 receptors.
Pharmacokinetics
Granisetron is a selective inhibitor of type 3 serotonergic (5-HT3) receptors. Granisetron has little or no affinity for other serotonin receptors, including 5-HT 1 , 5-HT 1A , 5-HT 1B/C , or 5-HT 2 ; for alpha 1 -, alpha 2 -, or beta-adrenoreceptors; for dopamine D 2 receptors; for histamine H 1 receptors; for benzodiazepine receptors; for picrotoxin receptors; or for opioid receptors. In most human studies, granisetron has had little effect on blood pressure, heart rate, or electrocardiogram (ECG). The drug is structurally and pharmacologically related to ondansetron, another selective inhibitor of 5-HT3 receptors. The serontonin 5-HT3 receptors are located on the nerve terminals of the vagus in the periphery, and centrally in the chemoreceptor trigger zone of the area postrema. The temporal relationship between the emetogenic action of emetogenic drugs and the release of serotonin, as well as the efficacy of antiemetic agents suggest that chemotherapeutic agents release serotonin from the enterochromaffin cells of the small intestine by causing degenerative changes in the GI tract. The serotonin then stimulates the vagal and splanchnic nerve receptors that project to the medullary vomiting center, as well as the 5-HT3 receptors in the area postrema, thus initiating the vomiting reflex, causing nausea and vomiting.
Clinical Use
Prevention or treatment of nausea and vomiting induced by cytotoxic chemotherapy, radiotherapy, or postoperative nausea and vomiting (PONV)
Side Effects
This causes vagal afferent discharge, inducing vomiting. In binding to 5-HT3 receptors, granisetron blocks serotonin stimulation, hence vomiting, after emetogenic stimuli such as cisplatin. Headache is the most frequently reported adverse effect of these medications.
Drug interactions
Potentially hazardous interactions with other drugs
Cytotoxics: possible increased risk of ventricular
arrhythmias with panobinostat.
Metabolism
Primarily hepatic; undergoes N -demethylation and aromatic ring oxidation followed by conjugation. Animal studies suggest that some of the metabolites may have 5-HT 3 receptor antagonist activity.
Metabolism
Granisetron is metabolised primarily in the liver by oxidation followed by conjugation. The major compounds are 7-OH-granisetron and its sulphate and glycuronide conjugates. Although antiemetic properties have been observed for 7-OH-granisetron and indazoline N-desmethyl granisetron, it is unlikely that these contribute significantly to the pharmacological activity of granisetron in man. Clearance is predominantly by hepatic metabolism. Urinary excretion of unchanged granisetron averages 12% of dose whilst that of metabolites amounts to about 47% of dose. The remainder is excreted in faeces as metabolites.
Storage
+4°C (desiccate)
Properties of Granisetron
| Boiling point: | 532.0±40.0 °C(Predicted) |
| Density | 1.33±0.1 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | 25℃: DMSO |
| form | Powder |
| pka | 12.34±0.20(Predicted) |
| color | White to off-white |
| InChI | InChI=1/C18H24N4O/c1-21-13-6-5-7-14(21)11-12(10-13)19-18(23)17-15-8-3-4-9-16(15)22(2)20-17/h3-4,8-9,12-14H,5-7,10-11H2,1-2H3,(H,19,23)/t12-,13+,14-/i2D3 |
Safety information for Granisetron
Computed Descriptors for Granisetron
| InChIKey | MFWNKCLOYSRHCJ-JQHPXDLNNA-N |
| SMILES | C1(C(=O)N[C@H]2C[C@@]3([H])CCC[C@@]([H])(C2)N3C)C2C=CC=CC=2N(C([2H])([2H])[2H])N=1 |&1:4,6,11,r| |
Granisetron manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran






You may like
-
 109889-09-0 Granisetron 98%View Details
109889-09-0 Granisetron 98%View Details
109889-09-0 -
 109889-09-0 98%View Details
109889-09-0 98%View Details
109889-09-0 -
 Granisetron 99%View Details
Granisetron 99%View Details -
 Granisetron Base 109889-09-0 99%View Details
Granisetron Base 109889-09-0 99%View Details
109889-09-0 -
 109889-09-0 / 121061-98-1 Granisetron 98%View Details
109889-09-0 / 121061-98-1 Granisetron 98%View Details
109889-09-0 / 121061-98-1 -
 Granisetron 99%View Details
Granisetron 99%View Details
109889-09-0 / 121061-98-1 -
 109889-09-0 / 121061-98-1 Granisetron 98%View Details
109889-09-0 / 121061-98-1 Granisetron 98%View Details
109889-09-0 / 121061-98-1 -
 Granisetron CASView Details
Granisetron CASView Details
