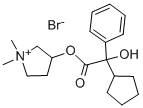
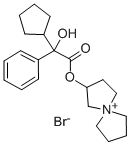
Glycopyrrolate
Synonym(s):Glycopyrrolate;glycopyrronium bromide;α-cyclopentylmandelic acid ester with 3-hydroxy-1,1-dimethylpyrrolidinium bromide;1-methyl-3-pyrrolidyl α-cyclopentylmandelate methobromide;1-methyl-3-pyrrolidyl α-phenyl-α-cyclopentylglycolate methobromide
- CAS NO.:596-51-0
- Empirical Formula: C19H28BrNO3-
- Molecular Weight: 398.33
- MDL number: MFCD00072137
- EINECS: 209-887-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-11 08:41:34
What is Glycopyrrolate?
Description
Glycopyrrolate is an antagonist of muscarinic acetylcholine receptors (mAChRs; Kis = 0.42, 1.77, 0.52, 0.78, and 1.29 nM for the M1-M5 receptors, respectively). It induces relaxation of precontracted isolated human bronchi when used at concentrations of 0.01, 0.1, or 1 μM. Glycopyrrolate reduces post-prandial gastric antral motility in dogs when administered at a dose of 0.01 mg/kg. It inhibits salivation in a rat model of sialorrhea induced by pilocarpine with an ED50 value of 0.74 μg/kg. Formulations containing glycopyrrolate have been used in the treatment of sialorrhea, peptic ulcers, and chronic obstructive pulmonary disease (COPD).
Chemical properties
White Solid
Originator
Robinul,Robins,US,1961
The Uses of Glycopyrrolate
Inhaled glycopyrrolate treats air flow blockage and prevents the worsening chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema. COPD is a long-term lung disease that causes bronchospasm (difficulty with breathing). It is a synthetic, quaternary ammonium anticholinergic. Antispasmodic; preanesthetic medicant. For use as a preoperative antimuscarinic to reduce salivary, tracheobronchial, and pharyngeal secretions, to reduce the volume and free acidity of gastric secretions and to block cardiac vagal inhibitory reflexes during induction of anaesthesia and intubation. Glycopyrrolate inhibits the secretion of digestive juices and restores normal stomach function. It is used for treating stomach ulcers and inflamed intestines and as a pre-operational drug for inhibiting excess stomach secretion.
Definition
ChEBI: A quaternary ammonium salt composed of 3-{[cyclopentyl(hydroxy)phenylacetyl]oxy}-1,1-dimethylpyrrolidin-1-ium and bromide ions in a 1:1 ratio.
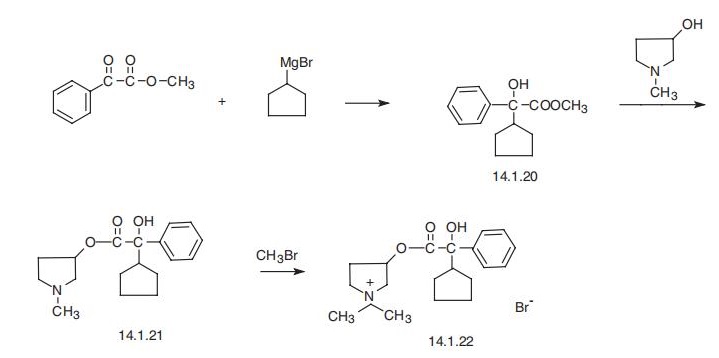
Manufacturing Process
A mixture of 42.5 grams (0.17 mol) of methyl α-cyclopentyl mandelate and 18
grams (0.175 mol) of 1-methyl-3-pyrrolidinol in 500 ml of heptane was
refluxed under a Dean and Stark moisture trap, with the addition of four 0.1
gram pieces of sodium at 1-hour intervals. After 5 hours' refluxing the
solution was concentrated to one-half volume, and extracted with cold 3N HCl.
The acid extract was made alkaline with aqueous sodium hydroxide and
extracted with ether which was washed, dried over sodium sulfate, filtered
and concentrated. The residue was fractionated at reduced pressure. Yield 33
grams (64%); BP 151° to 154°C/0.2 mm, nD23= 1.5265.
The hydrochloride salt was precipitated as an oil from an ethereal solution of
the base with ethereal hydrogen chloride. It was crystallized from butanone;
MP 170° to 171.5°C.
The methyl bromide quaternary was prepared by saturating a solution of the
base in dry ethyl acetate with methyl bromide. After standing for 9 days the resulting crystalline solid was filtered and recrystallized from butanone and
from ethyl acetate; MP 193° to 194.5°C.
brand name
Robinul (Baxter Healthcare); Robinul (Sciele).
Therapeutic Function
Spasmolytic
General Description
Glycopyrrolate, 3-hydroxy-1,1-dimethylpyrrolidinium bromide -cyclopentyl mandelate (Robinul), occurs as a white, crystalline powder that is soluble in water or alcohol but is practically insoluble in chloroform or ether.
Glycopyrrolate is a typical anticholinergic and possesses, at adequate dosage levels, the atropine-like effects characteristic of this class of drugs. It has a spasmolytic effect on the musculature of the GI tract as well as the genitourinary tract. It diminishes gastric and pancreatic secretions and the quantity of perspiration and saliva. Its side effects are also typically atropine-like (i.e., dryness of the mouth, urinary retention, blurred vision, constipation). Glycopyrrolate is a more potent antagonist on M1 than on M2 and M3 receptors. The low affinity of M2 receptors may partly explain the low incidence of tachycardia during the use of this drug as an antispasmodic. Because of its quaternary ammonium character, glycopyrrolate rarely causes CNS disturbances, although, in sufficiently high dosage, it can bring about ganglionic and myoneural junction block.
Biochem/physiol Actions
Glycopyrrolate is long-acting muscarinic antagonist (LAMA). It is kinetically selective muscarinic M3 receptor antagonist.
Mechanism of action
Glycopyrrolate exhibits onset of action within 1 minute when given intravenously and an elimination half-life of approximately 50 minutes. Glycopyrrolate undergoes urinary excretion and elimination.
Clinical Use
Glycopyrrolate is used as an adjunct in the management of pepticulcer and other GI ailments associated with hyperacidity,hypermotility, and spasm. In common with other anticholinergics,its use does not preclude dietary restrictions or use ofantacids and sedatives if these are indicated.
Side Effects
- dry mouth
- blurred vision
- vision problems
- loss of taste
- headache
- nervousness
- confusion
- drowsiness
Safety Profile
Poison by intravenous and intraperitoneal routes. Moderately toxic by ingestion and subcutaneous routes. Experimental reproductive effects. When heated to decomposition it emits very toxic fumes of NOx and Br-. See also BROMIDES.
Synthesis
Glycopyrrolate, 3-[(cyclopentylhydroxyphenylacetyl)oxy]-1,1-dimethyl-pyrrolidinium bromide, is synthesized from the methyl ester of α-cyclopentylmandelic acid by transesterification using 3-hydroxy-1-methylpyrrolidine as an alcohol component, which forms the ester, which is further transformed into a quaternary salt upon reaction with methylbromide, giving glycopyrrolate. The starting methyl ester of α-cyclopentyl mandelic acid is synthesized by reacting cyclopentyl magnesium bromide with the methyl ester of phenyl glyoxylic acid.
Veterinary Drugs and Treatments
Glycopyrrolate injection is approved for use in dogs and cats. The FDA approved indication for these species is as a preanesthetic anticholinergic agent. The drug is also used to treat sinus bradycardia, sinoatrial arrest, and incomplete AV block, where anticholinergic therapy may be beneficial. When cholinergic agents such as neostigmine or pyridostigmine are used to reverse neuromuscular blockade due to non-depolarizing muscle relaxants, glycopyrrolate may be administered simultaneously to prevent the peripheral muscarinic effects of the cholinergic agent.
Properties of Glycopyrrolate
| Melting point: | 192-195°C |
| Density | 1.3222 (rough estimate) |
| refractive index | 1.6200 (estimate) |
| storage temp. | 2-8°C |
| solubility | H2O: ≥24mg/mL |
| form | powder |
| color | white to tan |
| Merck | 14,4501 |
| Stability: | Hygroscopic |
| InChI | InChI=1S/C19H28NO3.BrH/c1-20(2)13-12-17(14-20)23-18(21)19(22,16-10-6-7-11-16)15-8-4-3-5-9-15;/h3-5,8-9,16-17,22H,6-7,10-14H2,1-2H3;1H/q+1;/p-1 |
| CAS DataBase Reference | 596-51-0(CAS DataBase Reference) |
Safety information for Glycopyrrolate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Glycopyrrolate
| InChIKey | VPNYRYCIDCJBOM-UHFFFAOYSA-M |
| SMILES | C(O)(C(=O)OC1CC[N+](C)(C)C1)(C1=CC=CC=C1)C1CCCC1.[Br-] |
Glycopyrrolate manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 596-51-0 98%View Details
596-51-0 98%View Details
596-51-0 -
 Glycopyrrolate/Glycopyronium Bromide - Micronised (D90 < 6 micron, D99 < 10 micron) 98%View Details
Glycopyrrolate/Glycopyronium Bromide - Micronised (D90 < 6 micron, D99 < 10 micron) 98%View Details
596-51-0 -
 596-51-0 GLYCOPYRROLATE -IP 99%View Details
596-51-0 GLYCOPYRROLATE -IP 99%View Details
596-51-0 -
 596-51-0 98%View Details
596-51-0 98%View Details
596-51-0 -
 Glycopyrrolate 99%View Details
Glycopyrrolate 99%View Details
596-51-0 -
 GLYCOPYRROLATE 596-51-0 95-99%View Details
GLYCOPYRROLATE 596-51-0 95-99%View Details
596-51-0 -
 Glycopyrrolate CAS 596-51-0View Details
Glycopyrrolate CAS 596-51-0View Details
596-51-0 -
 Glycopyrrolate CAS 596-51-0View Details
Glycopyrrolate CAS 596-51-0View Details
596-51-0
