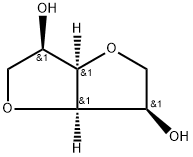
glucurono-1,4-lactone isonicotinoylhydrazone
- CAS NO.:3691-74-5
- Empirical Formula: C12H13N3O6
- Molecular Weight: 295.24812
- MDL number: MFCD01725299
- EINECS: 223-005-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:38
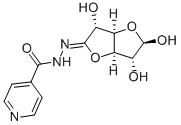
What is glucurono-1,4-lactone isonicotinoylhydrazone?
Originator
Gatalone,Barnes-Hind
Definition
ChEBI: Glyconiazide is an aromatic carboxylic acid and a pyridinemonocarboxylic acid.
Manufacturing Process
2 Methods of producing of d-glucuronolactone isonicotinyl hydrazone:
1. To 88.0 g of D-glucuronolactone, in the bottom of a 3 L round bottom flask,
was added 1.5 liters of methyl alcohol (acetone-free). The mixture was boiled
gently on a steam bath for 10 min, producing a clear solution. To this hot
solution, 70.0 g of isonicotinic acid hydrazide was added all at once. The
mixture was then boiled vigorously for 10 min and the clear solution filtered
without suction through a piece of lens paper into a two liter Erlenmeyer flask.
After the flask had been allowed to stand 24 h at room temperature, crystals
in the form of beautiful white rods and narrow plates were observed. These
crystals were filtered with suction, washed with a small amount of methyl
alcohol, and sucked to complete dryness. The resulting product was dried in a
vacuum desiccator for 3 days. Actual yield was 148.0 g, (yield of better than
99%). On heating the d-glucuronolactone isonicotinyl hydrazone thus formed,
the crystals charred and decomposed with foaming between 150° and 160°C
without any sharp melting point. The new compound was already very pure,
and upon recrystallization from a large amount of methyl or ethyl alcohol
(absolute) showed no appreciable change in physical properties from the
unrecrystallized product.
2. D-Glucuronic acid was liberated from a solution of its sodium salt (12.0 g)
in water (25 ml) by the addition of concentrated hydrochloric acid (5 ml). To
the mixture, sodium acetate (5.0 g) was added to remove the excess of the
mineral acid. Isonicotinic acid hydrazide (7.0 g) was then introduced into the
clear solution and mixing accomplished by thorough shaking. Methyl alcohol
(250 ml) was added and the mixture boiled on a steam bath for 10 min. A
white crystalline precipitate started to separate from the solution after a few
minutes heating and was allowed to stand overnight at room temperature
before filtering. The crystals (white small rods) were sucked to dryness,
washed with a small amount of absolute methanol and again sucked to
dryness. The product was further dried in a vacuum desiccator for 24 h. Yield:
25-50%. The hydrazone melted at 150°-160°C (dec.).
Therapeutic Function
Antitubercular
Properties of glucurono-1,4-lactone isonicotinoylhydrazone
| Melting point: | -23 °C |
| Boiling point: | 436.94°C (rough estimate) |
| Density | 1.3551 (rough estimate) |
| refractive index | 1.5290 (estimate) |
| pka | 11.20±0.46(Predicted) |
Safety information for glucurono-1,4-lactone isonicotinoylhydrazone
Computed Descriptors for glucurono-1,4-lactone isonicotinoylhydrazone
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Mefenamic Acid IP/BP/EP/USP Diclofenac Sodium IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4