Glaucarubin
- CAS NO.:1448-23-3
- Empirical Formula: C25H36O10
- Molecular Weight: 496.549
- MDL number: MFCD01745359
- EINECS: 815-051-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-26 15:15:06
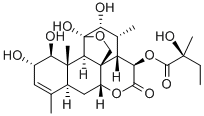
What is Glaucarubin?
Originator
Glarubin,Massengill,US,1959
Definition
ChEBI: Glaucarubin is a triterpenoid.
Manufacturing Process
The preparation of pure glaucarubin from Aceituno meal is conveniently
carried out by extracting the Aceituno meal with water, using about 100
gallons of the water per hundred pounds of meal. If the meal is in the form of
a relatively solid cake, it should be soaked in the water for a time to cause
disintegration. The temperature of the water is then raised to about 70°C for
the actual extraction, and the mixture is moderately agitated, while
maintaining a temperature of about 70°C for a period of about three hours,
until extraction is substantially complete. If desired, the extraction may be
conducted at lower temperatures down to about room temperature although
at such lower temperatures, the extraction is much slower and less efficient at
temperatures substantially higher than 70°C, there may be partial destruction
or decomposition of the product being recovered.
The slurry or extraction mixture is filtered while hot, and the resulting filter
cake is washed with about five to ten gallons of hot water; the primary filtrate
and wash water are combined and held for further processing. In order to
insure complete extraction of the desired material, the filter cake is again
extracted with about 100 gallons of water at 70°C. Although not essential, it is desirable to add to the second extraction a small quantity of acetic acid.
The acetic acid appears to aid in obtaining a complete and thorough
extraction. After extraction for about three hours with agitation at a
temperature of about 70°C, the slurry is again filtered and the cake washed
as before with about five to ten gallons of hot water. The resulting filtrate and
wash are then combined with the primary filtrate and wash.
The combined filtrates or total aqueous extracts are cooled to about room
temperature and filtered to remove any residual solids from solution. The
clarified aqueous extract is then concentrated to about 70 gallons at a
temperature below about 50°C, thus reducing the volume to about one-third
the original volume. The resulting concentrate is cooled to room temperature
or below and filtered to remove any tar or gum that may have separated. The
presence of tar or gum at this stage of the process will vary depending upon
the starting material and the manner in which the primary extraction has
been carried out. It has been found, however, that unless any tar or gum
present in the initial extract is removed by the procedure described, it will
seriously interfere with the further concentration and crystallization steps
hereinafter described.
After removal of such tar or gum, the concentrate is further evaporated at a
temperature below about 50°C to about one-fourth the volume, i.e., 70
gallons is concentrated to about 15 to 20 gallons. This concentrate is cooled
to a temperature of about 0°C to 5°C and allowed to stand for an extended
period, such as overnight, whereupon there is a separation of crude crystalline
glaucarubin therefrom. The crude crystals thus formed are removed by
filtration and the mother liquors again concentrated to about one-half volume
and cooled to permit separation of a second batch of crude glaucarubin
crystals. The two batches of crude glaucarubin crystals are combined and
dried preparatory to further purification.
The crude glaucarubin crystals obtained as above described from 100 pounds
of Aceituno meal are slurried with about sevenandanehalf gallons of
anhydrous methanol and refluxed until the crystals dissolve. The hot solution
is then filtered and the resulting filter cake washed with methanol. The filter
cake is then again extracted with an additional seven-and-one-half gallon
quantity of anhydrous methanol in the manner described, and filtered. The
methanol filtrates and washes are combined and concentrated at atmospheric
pressure until crystals begin to appear, i.e., generally after concentration to
about one-fifteenth volume. The solution is then cooled to about 0°C to 50°C
and allowed to stand for crystallization to go substantially to completion. The
resulting crystals are filtered off and the mother liquors are further
concentrated and cooled to collect a second crop of crystals. The two crops of
crystals are then combined and may be further purified by redissolving in
methanol, filtering through activated charcoal, and recrystallizing after
concentration of the methanol filtrate.
The purified crystalline glaucarubin thus obtained is colorless and odorless and
is estimated to have a purity of about 96% to 97%. It has the formula
C25H36O10 and melts at 262°C to 263°C with decomposition (capillary tube).
Therapeutic Function
Amebicidal
Properties of Glaucarubin
| Melting point: | 252.5°C (rough estimate) |
| Boiling point: | 508.86°C (rough estimate) |
| alpha | D25 +45° (c = 1.7 in pyridine); D25 +69° (c = 0.6 in methanol) |
| Density | 1.2066 (rough estimate) |
| refractive index | 1.5376 (estimate) |
Safety information for Glaucarubin
Computed Descriptors for Glaucarubin
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1