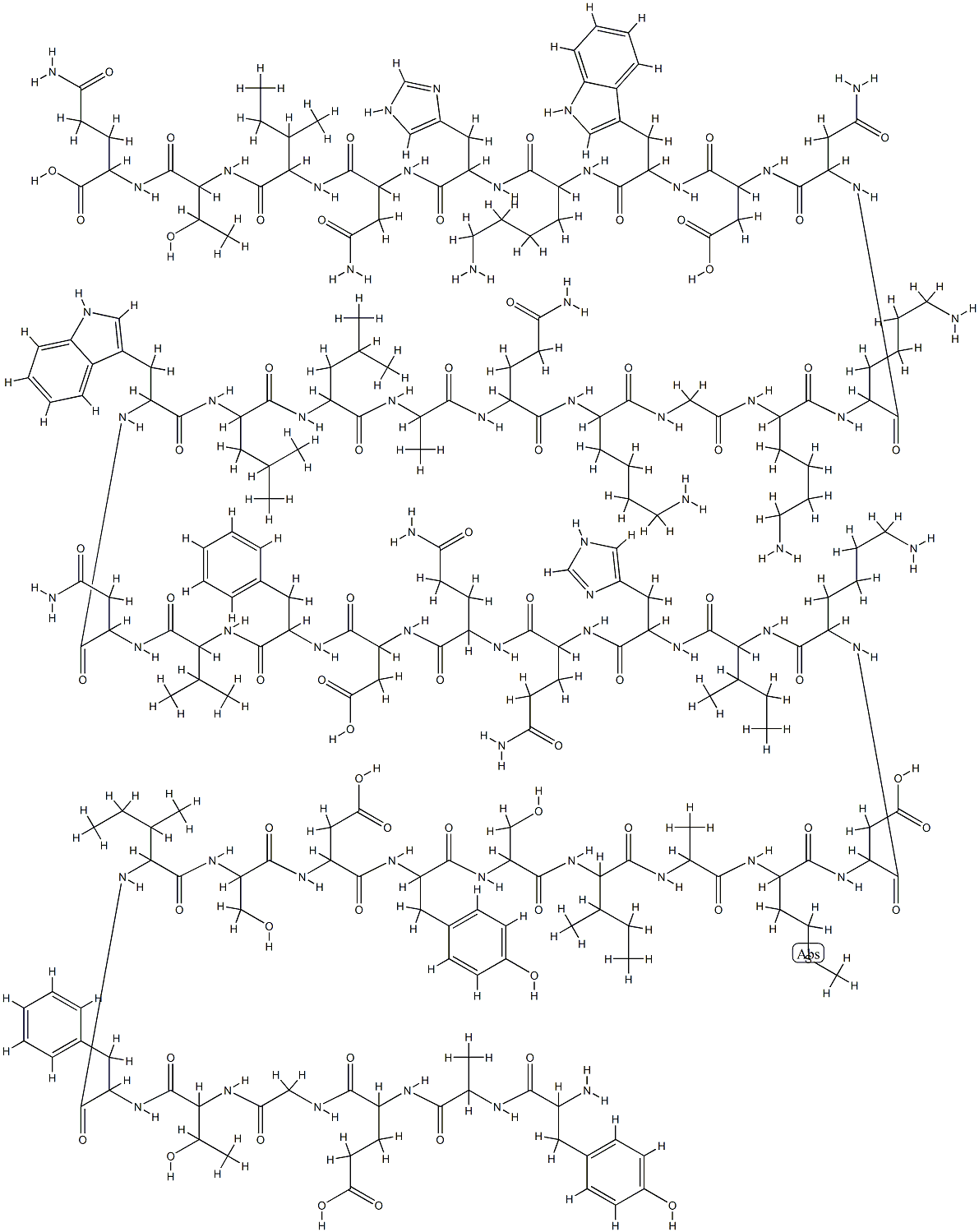
GIP (HUMAN)
Synonym(s):GIP;Glucose-dependent Insulinotropic Peptide;Tyr-Ala-Glu-Gly-Thr-Phe-Ile-Ser-Asp-Tyr-Ser-Ile-Ala-Met-Asp-Lys-Ile-His-Gln-Gln-Asp-Phe-Val-Asn-Trp-Leu-Leu-Ala-Gln-Lys-Gly-Lys-Lys-Asn-Asp-Trp-Lys-His-Asn-Ile-Thr-Gln
- CAS NO.:100040-31-1
- Empirical Formula: C226H338N60O66S
- Molecular Weight: 4983.52932
- MDL number: MFCD00081634
- Update Date: 2024-10-23 13:36:13
What is GIP (HUMAN)?
Description
GIP was originally isolated as a gastric inhibitory polypeptide. After the discovery of its glucose-dependent insulinotropic activity, known as the incretin effect, GIP was renamed glucose-dependent insulinotropic peptide.
Gastric inhibitory peptide
Abbreviation: GIP
Additional names: gastric inhibitory polypeptide,gastrointestinal inhibitory peptide, glucose-dependent, insulinotropic peptide
Chemical properties
Human GIP: Mr 4983.6, theoretical pI 6.92. GIP is soluble in water, but insoluble in ethanol. GIP is inactivated by DPP-4.
History
GIP was originally isolated from the porcine intestinal extract on the basis of its acid inhibitory activity in dogs (gastric inhibitory polypeptide) in the early 1970s, and subsequently renamed glucose-dependent insulinotropic peptide after the finding of its physiologically important role as a potentiator of glucose-stimulated insulin secretion.
The Uses of GIP (HUMAN)
GIP possessing incretin activity enhances glucosestimulated insulin release. GIP agonists are potentially useful for the treatment of diabetes. Moreover, DPP-4 inhibitors are approved for use in diabetes patients because GIP is rapidly deactivated by DPP-4.
What are the applications of Application
GIP (HUMAN) is a hormone that induces insulin secretion in response to glucose.
Biosynthesis
The expression of GIP is regulated by nutrients. The administration of glucose and lipid into the rat gastrointestinal tract increases GIP mRNA levels. Circulating GIP levels are low in the fasted state and increase within minutes of food ingestion. The postprandial levels of circulating GIP are dependent on meal size. The degree to which nutrients regulate GIP secretion is speciesdependent. Fat is a more potent stimulator than carbohydrates in humans, whereas in the rodent and pig, carbohydrates are more potent than fat. Once released, GIP is rapidly deactivated by DPP-4.
Receptors
Structure and subtype
The receptor of GIP is a seven-transmembrane GPCR that belongs to a subclass of the family B. Both the relatively long extracellular N-terminal domain and the first transmembrane domain are important for ligand binding and receptor activation. The C-terminal cytoplasmic domain of the receptor is important for receptor desensitization and internalization. Like their peptide ligands, the GIP receptor and the GLP-1 receptor exhibit high degrees of amino acid sequence identity, with similar molecular structures and signaling processes. However, GIP does not bind to the GLP-1 receptor and vice versa.
Signal transduction pathway
Ligand binding to the GIP receptor primarily activates adenylyl cyclase and increases intracellular cAMP.6 The activation of the MAP kinase pathway, the phospholipase A2 pathway, and the phosphatidylinositol 3-kinase/protein kinase B pathway have also been reported.
Agonist
[D-Ala2]-GIP is an agonist. Tirzepatide (LY3298176) is a dual agonist of GIP and GLP-1 receptors.
Antagonists
GIP(6–30), ANTGIP (GIP-(7–30)-NH2) (a truncated GIP peptide antagonist), GIP(3–30)NH2 (a truncated GIP peptide antagonist), and [Pro3]-GIP; Exendin(9–39) amide are antagonists.
storage
Store at -20°C
Structure and conformation
Human GIP is a single 42-aa peptide. The structure of vertebrate GIP is well conserved and both the N-terminal and central regions are important for biological activity because truncated forms of GIP, GIP(1–39), and GIP (1–30) show a high degree of biological activity.2 The N-terminal two aa residues are cleaved off by dipeptidyl-peptidase 4 (DPP-4) in the circulation to form GIP(3–42), which has no insulinotropic activity.
Properties of GIP (HUMAN)
| storage temp. | −20°C |
| form | Solid |
| color | White to off-white |
| Water Solubility | Soluble to 1 mg/ml in water |
Safety information for GIP (HUMAN)
Computed Descriptors for GIP (HUMAN)
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid Fmoc-Val-Cit-PAB DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonateRelated products of tetrahydrofuran

You may like
-
 Gastric Inhibitory Polypeptide human CAS 100040-31-1View Details
Gastric Inhibitory Polypeptide human CAS 100040-31-1View Details
100040-31-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8