GAMMA-SECRETASE INHIBITOR XII
- CAS NO.:161710-10-7
- Empirical Formula: C20H30N2O4
- Molecular Weight: 362.46
- MDL number: MFCD04974201
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 23:16:16
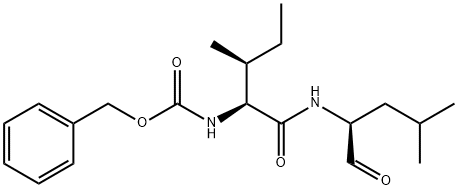
What is GAMMA-SECRETASE INHIBITOR XII?
Biological Activity
z-ile-leu-aldehyde (gamma-secretase inhibitor xii /gsi xii, z-il-cho) is a potent gamma-secretase inhibitor and notch signaling inhibitor.notch signaling impinges on many cellular processes, including cell proliferation, differentiation, apoptosis, and maintenance of stem cells. it is also involved in many developmental systems, including neurogenesis, angiogenesis, hematopoiesis, and myogenesis. deregulation of notch signaling leads to developmental syndromes and cancer [3]. in order to transmit a signal, notch receptors undergo a series of proteolytic cleavages after binding their cognate ligands of the delta-like and jagged family. γ-secretase is a large protease complex that cleaves the membrane-bound form of notch and releases the intracellular domain of the notch receptor. this step in notch signaling is pivotal in the activation of this signaling cascade [1] [2].ex vivo: a model of 3d culture of human breast cancer tissues was developed to study a series of 30 consecutive primary tumors from patients with untreated breast cancer for their sensitivity to the notch inhibitor gsi xii (15 μm). the results indicated that 24 tumors are sensitive to apoptosis induction by gsixii.
in vitro
gsi xii (10~15 μm) blocked the notch signaling, reduced cell viability and induced apoptosis in mopc315.bm murine multiple myeloma cells in vitro. gsi xii (10 μm) impaired murine osteoclast differentiation of receptor activator of nf-κb ligand (rankl)-stimulated raw264.7 murine monocyte/macrophage cell line [1]. gsi xii (8 μm ~ 15 μm) potently triggered an apoptotic response through inhibition of notch activity in the breast cancer cells. gsi xii also targets mammary cancer stem-like cells because it dramatically prevented in vitro mammosphere formation [3].
in vivo
notch inhibition through gsi xii (intraperitoneal injection, 10 mg/kg) reduced myeloma-specific paraprotein levels, bone loss and diminished osteolytic lesions in the mopc315.bm mice model[1]. notch inhibition using gsi xii (intraperitoneal injection, 5 mg/kg) blocked embryonal rhabdomyosarcoma tumorigenesis in mice[2].
References
[1]. schwarzer r, nickel n, godau j, et al. notch pathway inhibition controls myeloma bone disease in the murine mopc315. bm model[j]. blood cancer journal, 2014, 4(6): e217.
[2]. belyea b c, naini s, bentley r c, et al. inhibition of the notch-hey1 axis blocks embryonal rhabdomyosarcoma tumorigenesis[j]. clinical cancer research, 2011, 17(23): 7324-7336.
[3]. séveno c, loussouarn d, bréchet s, et al. γ-secretase inhibition promotes cell death, noxa upregulation, and sensitization to bh3 mimetic abt-737 in human breast cancer cells[j]. breast cancer research, 2012, 14(3): r96.
Properties of GAMMA-SECRETASE INHIBITOR XII
| Boiling point: | 545.6±45.0 °C(Predicted) |
| Density | 1.074±0.06 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | insoluble in EtOH; insoluble in H2O; ≥10.8 mg/mL in DMSO |
| form | solid |
| pka | 11.14±0.46(Predicted) |
| color | White to off-white |
Safety information for GAMMA-SECRETASE INHIBITOR XII
Computed Descriptors for GAMMA-SECRETASE INHIBITOR XII
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1