GALLIUM(III) CHLORIDE
Synonym(s):Gallium chloride;Gallium trichloride;Trichlorogallium
- CAS NO.:13450-90-3
- Empirical Formula: Cl3Ga
- Molecular Weight: 176.08
- MDL number: MFCD00011018
- EINECS: 236-610-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
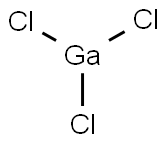
What is GALLIUM(III) CHLORIDE?
Chemical properties
white crystals or powder
Chemical properties
Gallium trichloride is a colorless solid which forms needle-like crystals. Acidic odor.
The Uses of GALLIUM(III) CHLORIDE
Gallium(III) chloride is a catalyst for a number of transformations including the ring opening and recyclization of epoxides and the trimerization of alkynes. It is used to detect the solar neutrions.
The Uses of GALLIUM(III) CHLORIDE
Lewis acid catalyst in organic synthesis; reagent for generating organogallium compounds, metallic gallium, and gallium semiconductors.
What are the applications of Application
Gallium(III) chloride is a transformation catalyst
Production Methods
The trichloride salt of trivalent gallium is prepared by the action of either hydrogen chloride or chlorine on the metal.
General Description
Colorless needles. Used as a raw material in the production of metallic gallium and in the processing of mono crystal semiconductor compounds.
Air & Water Reactions
Water soluble.
Reactivity Profile
GALLIUM(III) CHLORIDE acts as a weak inorganic acid. Materials in this group are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Health Hazard
(Non-Specific -- Gallium Compounds) In view of the toxicity of gallium and its compounds, as shown by experiments, all persons involved in work with these substances should undergo periodic medical examinations, during which special attention should be paid to the condition of the liver, respiratory organs, and skin.
Fire Hazard
Emits toxic chloride fumes when heated to decomposition. Decomposes upon sufficient heating.
Potential Exposure
Used as a raw material in the produc tion of metallic gallium; and in the processing of mono crystal, semiconductor compounds.
Shipping
UN3260 Corrosive solid, acidic, inorganic, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name Required. UN1759 Corrosive solids, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name required.
Purification Methods
The pure compound can be obtained by redistillation in a stream of Cl2 or Cl2/N2 followed by vacuum sublimation or zone refining. It forms colourless needles which give gallium dichloride [Ga(GaCl4), m 172.4o] on heating. It dissolves in H2O with liberation of heat. It is soluble in Et2O and can be extracted from an HCl solution with Et2O. [Laubengayer & Schirmer J Am Chem Soc 62 1579 1940, D.nges in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 846 1963.]
Properties of GALLIUM(III) CHLORIDE
| Melting point: | 78 °C(lit.) |
| Boiling point: | 35 °C |
| Density | 2.47 g/mL at 25 °C(lit.) |
| vapor pressure | 1 mm Hg ( 48 °C) |
| Flash point: | −26 °F |
| form | beads |
| color | White |
| Specific Gravity | 2.47 |
| Water Solubility | Very soluble in water. Soluble in benzene, carbon tetrachloride and carbon disulfide |
| Sensitive | Moisture Sensitive |
| Merck | 14,4348 |
| Stability: | Stability Stable, but reacts violently with water. |
| CAS DataBase Reference | 13450-90-3(CAS DataBase Reference) |
| EPA Substance Registry System | Gallium trichloride (13450-90-3) |
Safety information for GALLIUM(III) CHLORIDE
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for GALLIUM(III) CHLORIDE
| InChIKey | UPWPDUACHOATKO-UHFFFAOYSA-K |
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
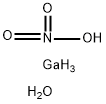
You may like
-
 Gallium (III) chloride, Ultra dry CAS 13450-90-3View Details
Gallium (III) chloride, Ultra dry CAS 13450-90-3View Details
13450-90-3 -
 Gallium (III) chloride, Ultra dry CAS 13450-90-3View Details
Gallium (III) chloride, Ultra dry CAS 13450-90-3View Details
13450-90-3 -
 Gallium (III) chloride, Ultra dry CAS 13450-90-3View Details
Gallium (III) chloride, Ultra dry CAS 13450-90-3View Details
13450-90-3 -
 Gallium(III) Chloride Anhydrous CAS 13450-90-3View Details
Gallium(III) Chloride Anhydrous CAS 13450-90-3View Details
13450-90-3 -
 Gallium(III) chloride CAS 13450-90-3View Details
Gallium(III) chloride CAS 13450-90-3View Details
13450-90-3 -
 Gallium(III) chloride, puriss CAS 13450-90-3View Details
Gallium(III) chloride, puriss CAS 13450-90-3View Details
13450-90-3 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
