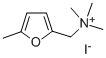
furtrethonium iodide
- CAS NO.:541-64-0
- Empirical Formula: C8H14INO
- Molecular Weight: 267.10733
- MDL number: MFCD00867181
- EINECS: 208-789-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
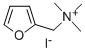
What is furtrethonium iodide?
Originator
Furmethide,SKF,US,1944
Manufacturing Process
Furfuryl dimethyl amine is first produced, This may conveniently be
accomplished by employing the Leuckart synthesis known to those skilled in
the art, which involves the use of an aldehyde or a ketone, and formate of
ammonia or an amine, or corresponding formamide derived by dehydration of
formate of ammonia or an amine.
For example, 5 mols of dimethyl amine and 5 mols of formic acid and water
are distilled to 135°C; distilling off most of the water. To the remaining liquid,
consisting for the most part of the formyl derivative of dimethyl amine, 1 mol
of furfural mixed with 1 mol of formic acid is slowly added with heating, the
temperature being maintained at 150°C to 170°C, until the reaction is
complete. The mixture is then distilled into a receiver. The course of this
reaction may be illustrated as follows:
Part of the formic acid used in the above reaction functions to react with the
dimethyl amine liberated in the reaction.
After the furfural has all been added and the reaction has subsided, the
residue is cooled, diluted with water, made strongly alkaline and distilled until
all volatile substances are removed, The distillate is then made acid with
formic acid and distilled with steam as long as nonbasic substances are
carried over by the steam. The residue is then made strongly basic with
caustic soda and the volatile amines again distilled with steam. The distillate
is then treated with strong alkali and then extracted with ether to extract the
base. The extract is dried by the addition of caustic potash, the ether removed
and the residual amine purified by distillation. Furfuryl dimethyl amine boils
over the range 145°C to 150°C.
To obtain the quaternary salt, furfuryl dimethyl amine so prepared is dissolved
in dry benzene and to the solution is added slightly more than one equivalent
of methyl iodide. Inducement of crystallization of the quaternary salt which
separates may be effected as, for example, by scratching the side of the
vessel containing the mixture or seeding with a small quantity of the
crystalline quaternary salt.
Therapeutic Function
Cholinergic
Properties of furtrethonium iodide
| Melting point: | 116-117° (Weilmuenster, Jordan); mp 118-120° (Khromov-Borisov) |
| storage temp. | -196°C |
Safety information for furtrethonium iodide
Computed Descriptors for furtrethonium iodide
New Products
ALUMINIUM IODIDE 100 GM BUFFER CAPSULE PH 7.0 - 10 CAP BUFFER SOLUTION PH 9.5 (BORATE) EZEE BLUE GEL STAINER BORAX CARMINE (GRENACHERS ALCOHOLIC) POTASSIUM IODATE - IODIDE SOLN 0.1 N Dabigatran Acyl-O3-D-Glucuronide Trifluoroacetic Acid Salt Isofolic Acid Dabigatran 2-O-acylglucuronide metabolite Dabigatran Acyl-?-D- glucuronide Trifluroacetic Acid Erythromycin EP Impurity A Desloratidine Related Compound ARelated products of tetrahydrofuran
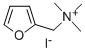


You may like
-
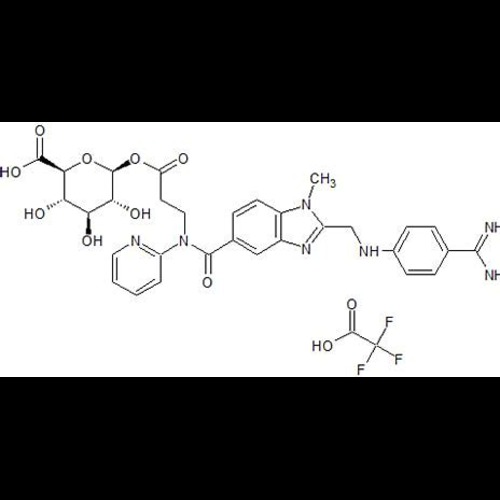 Dabigatran Acyl-O2-D-Glucuronide Trifluoroacetic Acid SaltView Details
Dabigatran Acyl-O2-D-Glucuronide Trifluoroacetic Acid SaltView Details -
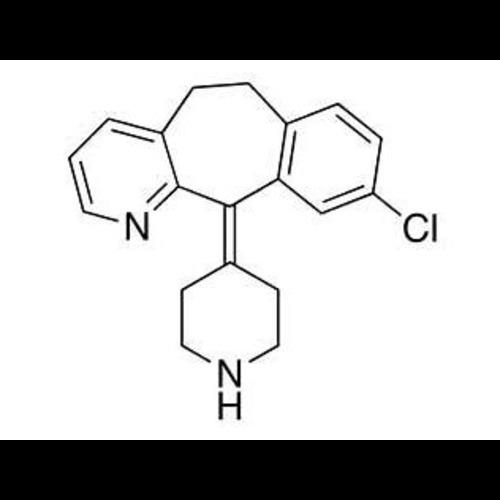 Dechloro DesloratadineView Details
Dechloro DesloratadineView Details -
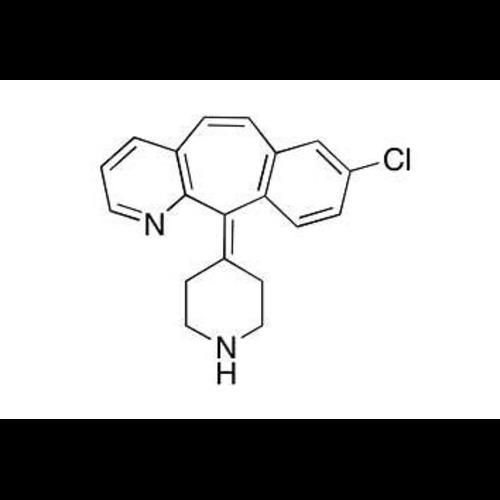 Dehydro DesloratadineView Details
Dehydro DesloratadineView Details -
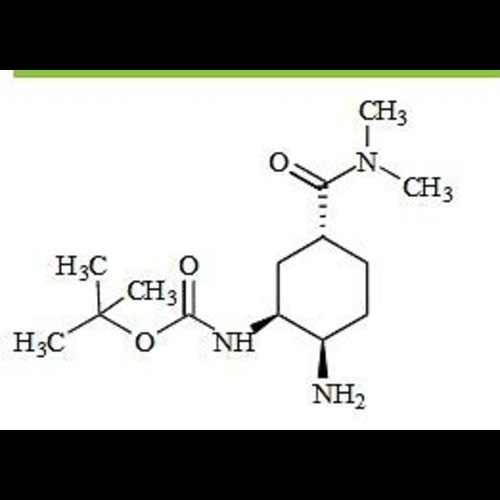 Edoxaban Impurity 57View Details
Edoxaban Impurity 57View Details
2089454-69-1 -
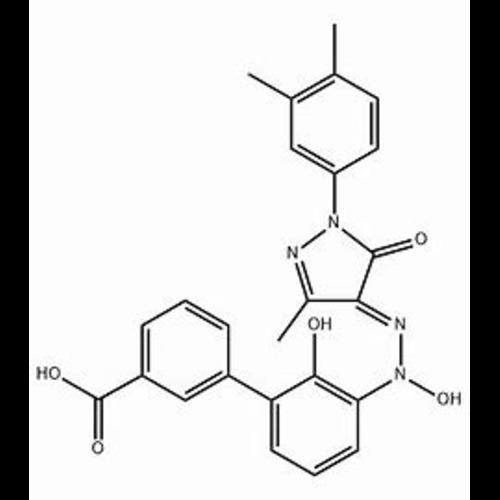 Eltrombopag N-Oxide ImpurityView Details
Eltrombopag N-Oxide ImpurityView Details
2734533-17-4 -
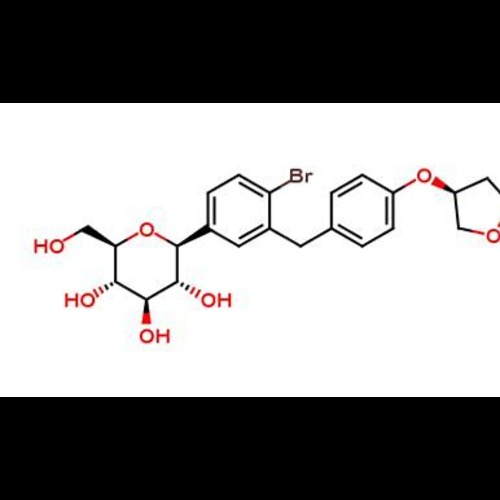 Empagliflozin Bromo ImpurityView Details
Empagliflozin Bromo ImpurityView Details -
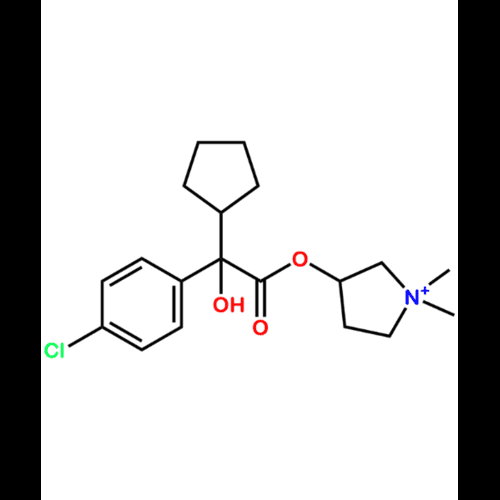 Glycopyrronium Bromide EP Impurity IView Details
Glycopyrronium Bromide EP Impurity IView Details
1404617-94-2 -
 Ipratropium EP Impurity BView Details
Ipratropium EP Impurity BView Details
58073-59-9