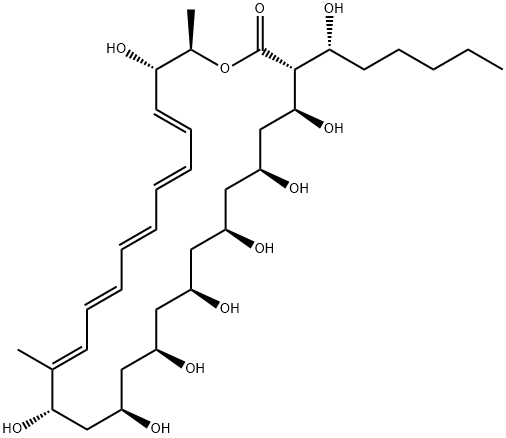
Fungichromin
- CAS NO.:6834-98-6
- Empirical Formula: C35H58O12
- Molecular Weight: 670.83
- EINECS: 229-913-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-02 08:54:58
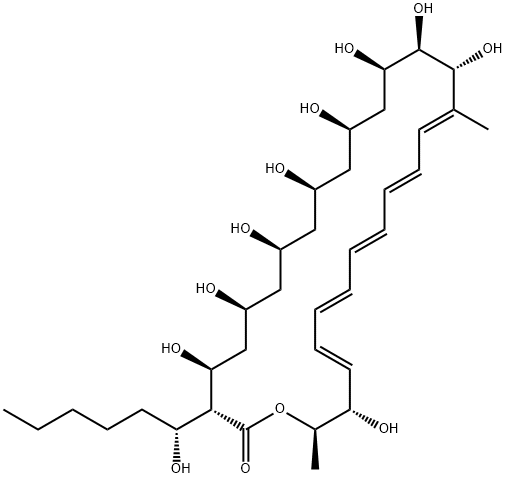
What is Fungichromin?
The Uses of Fungichromin
Lagosin is a pentaene antifungal produced by Streptomyces, first isolated in 1958 by researchers at MIT in the USA. The discovery was soon followed by several independent isolations as lagosin and cogomyin. Initially these metabolites were thought to be isomeric, but Pandey and colleagues at NCI definitively demonstrated they were identical. Structurally, lagosin is 14-hydroxyfilipin III and the most polar member of the filipin family of fungicides. Lagosin exhibits broad spectrum antifungal and antitumor activity and, like filipin, acts via interaction with cell membrane sterols.
Definition
ChEBI: Fungichromin is a macrolide and an antibiotic antifungal drug.
Biological Activity
lagosin, a polyene macrolide antibiotic, has first been extracted from an isolate of a streptomyces species present in a sample of soil collected in lagos, nigeria [1]. it has been reported that the three polyene macrolide antibiotics, fungichromin, lagosin, and cogomycin, showed some stereochemical differences at one or more centers [1].lagosin was the most polar member of the filipin family of fungicides with broad spectrum antifungal and antitumor activity, acting via interaction with cell membrane sterols. the antibiotic lagosin, which appeared to be identical to, or a stereoisomer of, fungichromin, and was very similar in structure to the filipins [2]. the equilibrium constants for association of the polyene antibiotics with aqueous suspensions of cholesterol follow the order filipin iil > amphotericin b > nystatin > lagosin, in agreement with the order reported for the extent of damage these antibiotics cause in natural and model membranes [2].
References
[1] pandey r c, guenther e c, aszalos a a, et al. physicochemical and biological comparison of polyene macrolide antibiotics fungichromin, lagosin and cogomycin[j]. the journal of antibiotics, 1982, 35(8): 988-996.
[2] bittman r, fischkoff s a. fluorescence studies of the binding of the polyene antibiotics filipin iii, amphotericin b, nystatin, and lagosin to cholesterol[j]. proceedings of the national academy of sciences, 1972, 69(12): 3795-3799.
Properties of Fungichromin
| Melting point: | 157-162° (dec) |
| Boiling point: | 914.0±65.0 °C(Predicted) |
| alpha | D20 -227.7° (c = 0.53 in DMF) |
| Density | 1.196±0.06 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| form | solid |
| pka | 12.77±0.70(Predicted) |
| color | Light yellow to yellow |
Safety information for Fungichromin
Computed Descriptors for Fungichromin
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4