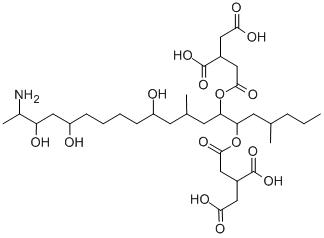
FUMONISIN B1
Synonym(s):FB1;Fumonisin B1 from Fusarium moniliforme;Macrofusin;Macrofusine;FB?
- CAS NO.:116355-83-0
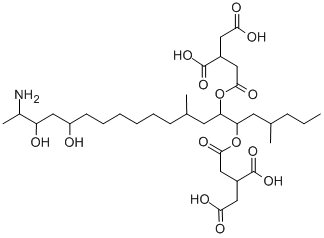
- Empirical Formula: C34H59NO15
- Molecular Weight: 721.83
- MDL number: MFCD00133349
- EINECS: 621-436-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-11 20:33:26
What is FUMONISIN B1?
Description
Fumonisin B1 (116355-83-0) inhibits ceramide synthesis (sphinganine N-acyltransferase), IC50 = 100 nM. Inhibits de novo sphingolipid biosynthesis (IC50 = 0.7 μM) thereby blocking glycosphingolipid production.
Chemical properties
white to tan powder
The Uses of FUMONISIN B1
Fumonisin B1 is a major analogue of a family of potent mycotoxins produced by various Fusarium species, associated with animal toxicity worldwide. In vitro, fumonisin B1 inhibits sphingosine N-acyl-transferase (ceramide synthase) and blocks the growth of axons.
The Uses of FUMONISIN B1
A mycotoxin produced by mold associated with corn. Fungal metabolite believed to cause leukoencephalomalacia in horses.
The Uses of FUMONISIN B1
A serine/threonine phosphatase inhibitor.
What are the applications of Application
Fumonisin B1 is a serine and threonine phosphatase inhibitor.
Definition
ChEBI: Fumonisin B1 is a diester that results from the condensation of the 1-carboxy groups of two molecules of propane-1,2,3-tricarboxylic acid with hydroxy groups at positions 14 and 15 of (2S,3S,5R,10R,12S,14S,15R,16R)-2-amino-12,16-dimethylicosane-3,5,10,14,15-pentol. It has a role as a metabolite and a carcinogenic agent. It is a fumonisin, a primary amino compound, a diester and a triol. It is functionally related to a (2S,3S,5R,10R,12S,14S,15R,16R)-2-amino-12,16-dimethylicosane-3,5,10,14,15-pentol. It is a conjugate acid of a fumonisin B1(3-).
General Description
Fumonisin B1 is a neurotoxin and a phytotoxin. A cell-permeable mycotoxin that inhibits sphingolipid biosynthesis in rat kidney and in liver microsomes by inhibition of sphingosine N-acyltransferase (ceramide synthase) (IC50 = 100 nM). Cellular effects also appear to be induced by micromolar levels of FB1. Because it inhibits ceramide synthase activity, it elevates cellular levels of sphingoid bases, including sphinganine, resulting in overall inhibition of sphingolipid biosynthesis. Preferentially inhibits sphingomyelin biosynthesis in neuronal cells. Has carcinogenic properties.
Biological Activity
Mycotoxin produced by Fusarium moniliforme . Potently inhibits sphingosine N-acyltransferase (ceramide synthase), causing an accumulation of sphingoid bases (IC 50 ~ 75 nM). Also inhibits protein phosphatases; IC 50 values are 80, 300, 400, 500 and 3000 μ M for PP5, PP2C α , PP2A, PP1 γ 2 and PP2B respectively.
Biochem/physiol Actions
Fumonisin B1 acts as a hepatocarcinogen and causes hepatotoxicity in rats. In horses, it causes leukoencephalitis, and in pigs, it causes pulmonary edema syndrome. In China and southern parts of Africa, it is linked with high incidence of esophageal cancer in humans. It acts as an inhibitor of sphingosine N-acyltransferase enzyme (ceramide synthase), related to structural similarities between fumonisin B1 and sphingoid bases like sphingosine.
storage
Store at +4°C
References
1) Merrill et al. (1993), Fumonisin B1 inhibits sphingosine (sphinganine) N-acyltransferase and de novo sphingolipid biosynthesis in cultured neurons in situ; J. Biol. Chem., 268 27299
Properties of FUMONISIN B1
| Melting point: | >60oC |
| Boiling point: | 713.81°C (rough estimate) |
| Density | 1.2207 (rough estimate) |
| refractive index | 1.6630 (estimate) |
| Flash point: | 6 °C |
| storage temp. | 2-8°C |
| solubility | Soluble in Water (up to 25 mg/ml). |
| pka | 3?+-.0.23(Predicted) |
| form | Powder |
| color | White to tan |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in distilled water may be stored at -20° for up to 3 months. |
| CAS DataBase Reference | 116355-83-0 |
| IARC | 2B (Vol. 56) 1993, 2B (Vol. 82) 2002 |
Safety information for FUMONISIN B1
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H351:Carcinogenicity H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. |
Computed Descriptors for FUMONISIN B1
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Fumonisin B1 from Fusarium moniliforme CAS 116355-83-0View Details
Fumonisin B1 from Fusarium moniliforme CAS 116355-83-0View Details
116355-83-0 -
 Fumonisin B1 solution CAS 116355-83-0View Details
Fumonisin B1 solution CAS 116355-83-0View Details
116355-83-0 -
 Fumonisin B1 CAS 116355-83-0View Details
Fumonisin B1 CAS 116355-83-0View Details
116355-83-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1