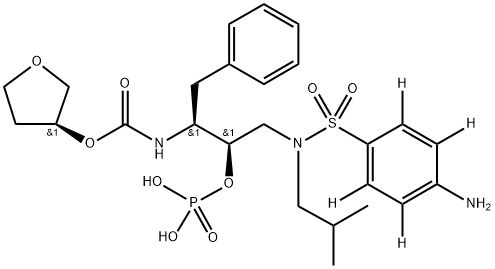
Fosamprenavir
- CAS NO.:226700-79-4
- Empirical Formula: C25H36N3O9PS
- Molecular Weight: 585.61
- MDL number: MFCD09955118
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-02 08:55:07
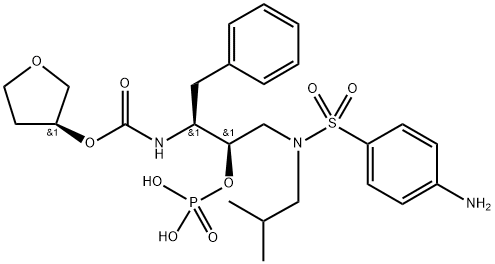
What is Fosamprenavir?
Absorption
The absolute oral bioavailability of amprenavir after administration of fosamprenavir in humans has not been established. After administration of a single 1,400 mg dose in the fasted state, fosamprenavir oral suspension (50 mg/mL) and fosamprenavir tablets (700 mg) provided similar amprenavir exposures (AUC), however, the Cmax of amprenavir after administration of the suspension formulation was 14.5 % higher compared with the tablet.
The Uses of Fosamprenavir
Antiviral (HIV protease inhibitor).
Indications
Indicated in combination with other antiretroviral agents for the treatment of human immunodeficiency virus (HIV-1) infection, as well as postexposure prophylaxis of HIV infection in individuals who have had occupational or nonoccupational exposure to potentially infectious body fluids of a person known to be infected with HIV when that exposure represents a substantial risk for HIV transmission. The use of fosamprenavir is pending revision due to a potential association between the drug and myocardial infarction and dyslipidemia in HIV infected adults.
Background
Fosamprenavir is a prodrug of amprenavir, an inhibitor of human immunodeficiency virus (HIV) protease.
Definition
ChEBI: A sulfonamide with a structure based on that of sulfanilamide substituted on the sulfonamide nitrogen by a (2R,3S)-4-phenyl-2-(phosphonooxy)-3-({[(3S)-tetrahydrofuran-3-yloxy]carbonyl}amino)butyl group. It is a pro-drug of the HIV protease inhibitor and antiretroviral drug amprenavir.
Pharmacokinetics
Fosamprenavir is hydrolyzed by cellular phosphatases to the antiretroviral protease inhibitor amprenavir. This hydrolysis allows for the slow release of amprenavir, reducing the number of pills a patient must take.
Metabolism
In the gut epithelium during absorption, fosamprenavir is rapidly and almost completely hydrolyzed to amprenavir and inorganic phosphate prior to reaching the systemic circulation. Amprenavir is metabolized in the liver by the cytochrome P450 3A4 (CYP3A4) enzyme system.
Properties of Fosamprenavir
| Density | 1.40±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO : ≥ 100 mg/mL (170.76 mM) |
| form | Solid |
| pka | 1.77±0.10(Predicted) |
| color | Light yellow to yellow |
Safety information for Fosamprenavir
Computed Descriptors for Fosamprenavir
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1