Forchlorfenuron
Synonym(s):N-(2-Chloro-4-pyridyl)-N′-phenylurea;4-CPPU;CPPU;Forchlorfenuron
- CAS NO.:68157-60-8
- Empirical Formula: C12H10ClN3O
- Molecular Weight: 247.68
- MDL number: MFCD00059898
- EINECS: 614-346-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
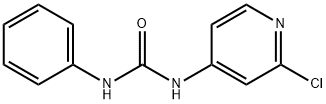
What is Forchlorfenuron?
Description
Forchlorfenuron (68157-60-8) reversibly perturbs mammalian septin assembly, organization and function.? Has no effect on actin or tubulin polymerization.1,2 FCF induces effects on mitosis and migration which phenocopy the effects induced by septin depletion using siRNA.1 A useful tool for exploring the physiological role of septin and its complexes.3,4
Description
August 3 is National Watermelon Day, and so this week’s molecules have a watermelon flavor.
Lycopene is the source of the red color in several fruits, notably watermelon (Citrullus lanatus?var.?lanatus) and tomato (Solanum lycopersicum). The pigment, also known as ψ,ψ-carotene, is a linear polyolefin that contains eight isoprene units. It has 13 double bonds, all in the?E-configuration.
In 1910, Swiss chemists Richard M. Willst?tter and Heinrich H. Escher isolated lycopene from tomatoes and determined its structure. But not until 1950 did another Swiss chemist, Paul Karrer, and his co-workers synthesize it.
Lycopene is an antioxidant that may have some health benefits, particularly against cancer and cardiovascular diseases. But agencies such as the European Food Safety Authority and the US Food and Drug Administration are not convinced; on the basis of current evidence, they have refused to label lycopene as a beneficial drug.
Forchlorfenuron, formally 1-(2-chloropyridin-4-yl)-3-phenylurea (CPPU), is a growth regulator that increases the size (and therefore the value) of such fruits as apple, cherry, and kiwi. It’s been used on watermelon too; but at least once, the results were unexpected.
In China in 2011, about 20 farmers applied too much CPPU on their watermelon crops too late in the growing season. The weather conditions were ideal, so much so that the watermelons grew too fast and exploded. The watermelon fragments, about 46 hectares worth, were used to feed fish and pigs.
Chemical properties
White Solid
Chemical properties
White to faint yellow crystalline powder.
The Uses of Forchlorfenuron
Forchlorfenuronis is a diphenylurea-derivative cytokinin. Forchlorfenuronis is used as a plant growth regulator (PGR). Forchlorfenuronis is commonly used in horticulture to stimulate the growth of kiw i fruit and grapes.
What are the applications of Application
Forchlorfenuron is a cytokinin that promotes cell division and cell differentiation
Definition
ChEBI: Forchlorfenuron is a member of the class of phenylureas that is urea substituted by a phenyl group and a 2-chloropyridin-4-yl group at positions 1 and 3 respectively. It is a plant growth regulator widely used in agriculture for improving fruit quality and fruit size. It has a role as a plant growth regulator. It is a member of phenylureas and a monochloropyridine.
Agricultural Uses
Plant growth regulator: Forchlorfenuron is as a plant growth regulator to promote cell division, and to improve the quality and the yield of fruits, especially table grapes, grape raisins, and kiwi fruit. In some parts of California, forchlorfenuron is said to double the size of Thompson seedless grapes and delay crop maturity up to several weeks. widely used in agriculture on fruits to increase their size,
Trade name
CN-11-3183; KT-30®; SKW 20010
Potential Exposure
Phenylurea/substituted urea plant growth regulator widely used in agriculture on fruits to increase their size, to promote cell division, and to improve the quality and the yield of fruits, especially table grapes, grape raisins, and kiwi fruit. In some parts of California, forchlorfenuron is reputed to double the size of Thompson seedless grapes and delay crop maturity up to several weeks.
Shipping
UN2767 Phenyl urea pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
May react with strong oxidizers such as chlorates, peroxides, nitrates, etc. Dust may form explosive mixture with air.
Waste Disposal
Containers must be disposed of properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. If this material cannot be disposed of according to label instruc tions, it may be dissolved or mixed with a combustible sol vent and burned in a chemical incinerator equipped with an afterburner and scrubber. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers.
References
1) Hu et al. (2008), Forchlorfenuron alters mammalian septin assembly, organization, and dynamics; J. Biol. Chem., 283 29563 2) DeMay et al. (2010), Cellular requirements for the small molecule forchlorfenuron to stabilize the septin cytoskeleton; Cytoskeleton, 67 383 3) Wasik et al. (2012), Septin 7 forms a complex with CD2AP and nephrin and regulates glucose transporter trafficking; Mol. Biol. Cell, 23 3370 4) Vardi-Oknin et al. (2013), Forchlorfenuron disrupts SEPT9_i1 filaments and inhibits HIF-1; PLoS One, 8(8) e73179
Properties of Forchlorfenuron
| Melting point: | 170-172°C |
| Boiling point: | 308.4±27.0 °C(Predicted) |
| Density | 1.415±0.06 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | Soluble in DMSO or Ethanol |
| form | neat |
| pka | 12.55±0.70(Predicted) |
| form | Solid |
| color | White or off-white |
| Stability: | Stable for 1 year from date ofpurchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 68157-60-8(CAS DataBase Reference) |
| EPA Substance Registry System | Forchlorfenuron (68157-60-8) |
Safety information for Forchlorfenuron
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H351:Carcinogenicity H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P391:Collect spillage. Hazardous to the aquatic environment P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. |
Computed Descriptors for Forchlorfenuron
| InChIKey | GPXLRLUVLMHHIK-UHFFFAOYSA-N |
Forchlorfenuron manufacturer
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran

You may like
-
 Forchlorfenuron 98% CAS 68157-60-8View Details
Forchlorfenuron 98% CAS 68157-60-8View Details
68157-60-8 -
 N-(2-Chloro-4-pyridyl)-N′-phenylurea CAS 68157-60-8View Details
N-(2-Chloro-4-pyridyl)-N′-phenylurea CAS 68157-60-8View Details
68157-60-8 -
 1-(2-Chloro-4-pyridyl)-3-phenylurea CAS 68157-60-8View Details
1-(2-Chloro-4-pyridyl)-3-phenylurea CAS 68157-60-8View Details
68157-60-8 -
 Forchlorfenuron (KT-30 CAS 68157-60-8View Details
Forchlorfenuron (KT-30 CAS 68157-60-8View Details
68157-60-8 -
 N-(2-Chloro-4-pyridyl)-N′-phenylurea CAS 68157-60-8View Details
N-(2-Chloro-4-pyridyl)-N′-phenylurea CAS 68157-60-8View Details
68157-60-8 -
 Forchlorfenuron CAS 68157-60-8View Details
Forchlorfenuron CAS 68157-60-8View Details
68157-60-8 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 609-15-4View Details
609-15-4View Details
609-15-4
