Fluralaner
- CAS NO.:864731-61-3
- Empirical Formula: C22H17Cl2F6N3O3
- Molecular Weight: 556.29
- EINECS: 200-001-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
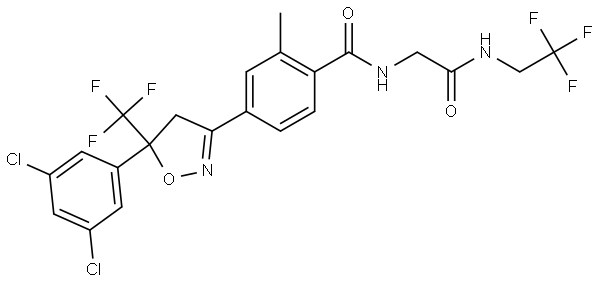
What is Fluralaner?
Description
Spring is finally here. Tennyson wrote, “In the Spring a young man’s fancy lightly turns to thoughts of love,” but these days, a pet owner’s fancy often turns to thoughts of controlling fleas on his or her furry friends.
Many treatments are in use to repel fleas (and ticks) from dogs and cats or to kill the insects once an infestation occurs: Treatments include topical powders and liquids, flea collars, and oral medications. All of these methods have advantages and disadvantages; the Internet is full of arguments for and against each.
In 2014, the US Food and Drug Administration approved the use of fluralaner (trade name Bravecto), a chewable tablet for dogs that is administered at 12-week intervals. This is a significant improvement to oral products that must be taken monthly. Fluralaner is a systemic insecticide that inhibits GABA-gated and l-glutamate-gated chloride channels in the insects’ nervous system.
FDA evidently considered the compounded product Bravecto to be safe enough for general use, but a glance at the hazard information box reveals that fluralaner itself is not to be handled carelessly. The dosage of fluralaner in Bravecto ranges from 112.5 mg to 1.4 g, depending on the weight of the dog to be treated. It can be inferred that even at the highest dose, Bravecto is deemed safe to be handled by the dog’s human companion.
Description
Fluralaner (INN) is a systemic insecticide and acaricide that is administered orally. The U.S. Food and Drug Administration (FDA) approved it under the trade name Bravecto for flea treatment in dogs in May 2014. The EU approved the drug in February 2014. Australia approved it for the treatment and prevention of ticks and fleas on dogs in January 2015.
The Uses of Fluralaner
Fluralaner is a novel systemically administered insecticidal and acaricidal compound that provides long-acting efficacy after oral administration to dogs. Fluralaner belongs to a new class of compounds, the isoxazolines. A field study has shown that a single fluralaner dose administered orally to dogs provides at least twelve weeks of flea- and tick-control. The long duration of activity offers a more convenient treatment over monthly flea and tick control treatments with a potential compliance advantage, reducing the risk of vector-transmitted diseases.
Indications
Fluralaner is an insecticide and acaricide used to treat Sarcoptes scabiei var. canis infestation in dogs. Flea treatment. A possible anti-malarial found to kill disease-spreading mosquitoes when they bite treated people.
Pharmacokinetics
Following oral administration, fluralaner is readily absorbed reaching maximum plasma concentrations within 1 day. Food enhances the absorption. Fluralaner is systemically distributed and reaches the highest concentrations in fat, followed by liver, kidney and muscle. The prolonged persistence and slow elimination from plasma (t1/2 = 12 days) and the lack of extensive metabolism provide effective concentrations of fluralaner for the duration of the inter-dosing interval. Individual variation in Cmax and t1/2 was observed. The major route of elimination is the excretion of unchanged fluralaner in faeces (~90% of the dose). Renal clearance is the minor route of elimination.
Side Effects
Most pets have very few side effects from fluralaner, provided it is given according to label recommendations and at the prescribed interval (or for off-label use, according to your veterinarian's directions). Side effects may include vomiting, diarrhea, decreased appetite, or flaky skin.
vcahospitals.com
Overdosage
No adverse reactions were observed following oral administration to puppies aged 8–9 weeks and weighing 2.0–3.6 kg treated with overdoses of up to 5 times the maximum recommended dose (56 mg, 168 mg and 280 mg fluralaner/kg bodyweight) on three occasions at shorter intervals than recommended (8-week intervals).
There were no findings on reproductive performance and no findings of concern on offspring viability when fluralaner was administered orally to Beagle dogs at overdoses of up to 3 times the maximum recommended dose (up to 168 mg/kg bodyweight of fluralaner).
The veterinary medicinal product was well tolerated in Collies with a deficient multidrug-resistanceprotein 1 (MDR1 -/-) following single oral administration at 3 times the recommended dose (168 mg/kg bodyweight). No treatment-related clinical signs were observed.
Drug interactions
Fluralaner is highly bound to plasma proteins and might compete with other highly bound drugs such as non-steroidal anti-inflammatory drugs (NSAIDs) and the cumarin derivative warfarin. Incubation of fluralaner in the presence of carprofen or warfarin in dog plasma at maximum expected plasma concentrations did not reduce the protein binding of fluralaner, carprofen or warfarin.
During clinical field testing, no interactions between Bravecto chewable tablets for dogs and routinely used veterinary medicinal products were observed.
Mode of action
Fluralaner is an inhibitor of the arthropod nervous system. It inhibits γ-aminobutyric acid (GABA)-gated chloride channels (GABAA receptors) and L-glutamate-gated chloride channels (GluCls). Potency of fluralaner is comparable to fipronil (a related GABA-antagonist insecticide and acaricide).
Properties of Fluralaner
| Melting point: | not available |
| Density | 1.51±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO:150.0(Max Conc. mg/mL);269.65(Max Conc. mM) Ethanol:25.0(Max Conc. mg/mL);44.94(Max Conc. mM) |
| solubility | insoluble |
| appearance | white to off-white crystals or powder |
| pka | 12.50±0.46(Predicted) |
| form | A crystalline solid |
| color | White to off-white |
Safety information for Fluralaner
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H360:Reproductive toxicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P281:Use personal protective equipment as required. P391:Collect spillage. Hazardous to the aquatic environment P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Fluralaner
| InChIKey | MLBZKOGAMRTSKP-UHFFFAOYSA-N |
| SMILES | C(NCC(=O)NCC(F)(F)F)(=O)C1=CC=C(C2CC(C3=CC(Cl)=CC(Cl)=C3)(C(F)(F)F)ON=2)C=C1C |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
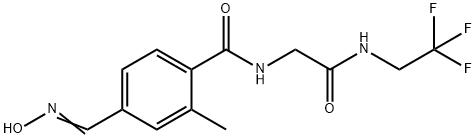
![4-[5-(3,5-dichlorophenyl)-5-(trifluoromethyl)-4,5-dihydro-1,2-oxazol-3-yl]-2-methylbenzoic acid](https://img.chemicalbook.in/CAS/20180529/GIF/864725-62-2.gif)
You may like
-
 864731-61-3 FluralanerView Details
864731-61-3 FluralanerView Details
864731-61-3 -
 864731-61-3 98%View Details
864731-61-3 98%View Details
864731-61-3 -
 Fluralaner cas No 864731-61-3View Details
Fluralaner cas No 864731-61-3View Details
864731-61-3 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
