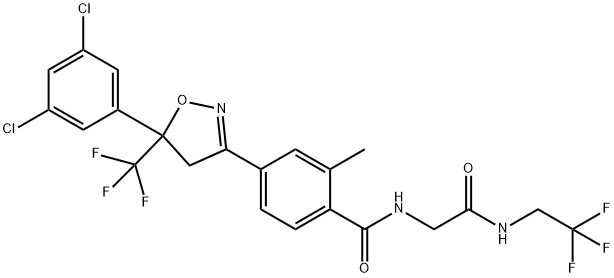
afoxolaner
- CAS NO.:1093861-60-9
- Empirical Formula: C26H17ClF9N3O3
- Molecular Weight: 625.87
- MDL number: MFCD30533411
- EINECS: 200-001-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-12 13:55:47
What is afoxolaner?
Description
Afoxolaner is an insecticide and acaricide belonging to the isoxazoline family. Afoxolaner acts at ligand-gated chloride channels, in particular those gated by the neurotransmitter gamma-aminobutyric acid (GABA), thereby blocking pre- and post-synaptic transfer of chloride ions across cell membranes.
The Uses of afoxolaner
Afoxolaner (brand name NexGard®) is used to treat and control flea and tick infestations in dogs. After being ingested by a dog, afoxolaner is distributed throughout the dog's body. When fleas or ticks bite the dog, they are exposed to the drug and killed during their blood meal.
Definition
Afoxolaner is used to treat and control flea and tick infestations in dogs. After being ingested by a dog, afoxolaner is distributed throughout the dog’s body. When fleas or ticks bite the dog, they are exposed to the drug and killed during their blood meal.
Pharmacokinetics
Afoxolaner orally administered to dogs was rapidly absorbed into blood. Max. blood levels were reached 2 to 12 hours after administration. Mean half-life in blood ranged from 7.7 to 17.8 days. Bioavailability was estimated to be approx. 74%. Half-life was higher in Collies than in Beagles, but no serious adverse effects were observed after treatment of Collies at 25 mg/kg bw (i.e. ~10x the therapeutic dose).Absorbed afoxolaner binds strongly (>99%) to blood proteins but it seems not to cause displacement of other drugs with high protein binding capacities.In dogs afoxolaner is slowly broken down into various metabolites, the major ones being a hydroxylate and a glucuronide. Elimination of both the metabolites and the parent compound occurrs mainly via biliary excretion and to a lesser extent through urine.
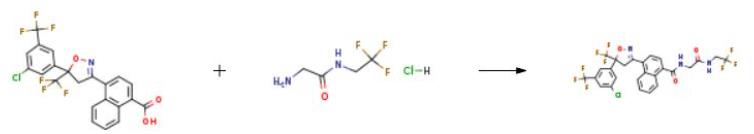
Synthesis
The synthesis of afoxolaner is as follows:
Add to the 100ml reaction flaskIntermediate 4 (8.57g), HBTU (8.65g, 22.38mmol), DMF (20ml),Intermediate 5, mix well and add triethylamine. Make the pH of the mixed liquid system=10,Stir at room temperature for 40 minutes, TLC monitors the progress of the reaction,After full reaction,Add 40ml of water, stir and crystallize,Suction filtration, wash the filter cake with 20ml water,Dry the filter cake under reduced pressure,Then use a mixture of ethyl acetate and petroleum ether(1:2) or ethanol and water (1:2)The mixture is recrystallized,The white powder product Aphrana 1-2, (8.10g, yield 77%, purity 99.47%) was obtained.
Enzyme inhibitor
This orally active isoxazoline-based pesticide (FW = 901.18 g/mol; CAS 1093861-60-9; IUPAC Name: 4-[5-[3-chloro-5-(trifluoromethyl)phenyl]- 4,5-dihydro-5-(trifluoromethyl)-3-isoxazolyl]-N-[2-oxo-2-[(2,2,2-trifluoroethyl) amino]ethyl]-1-naphthalenecarboxamide, is highly active against fleas (Ctenocephalides felis) and ticks (Dermacentor variabilis) in dogs. Afoxolaner is more potent, when tested in vitro, than any other compound ever tested in the membrane feeding system, including the avermectins. At an oral dose of 2.5 mg/kg, afoxolaner reaches pharmacologically effective plasma concentrations (0.1–0.2 μg/mL), blocking native and expressed insect GABA-gated chloride channels with nanomolar potency. Afoxolaner has comparable potency between wild-type channels as well as channelspossessing the dieldrin resistance-conferring Alanine-302-Serine mutation. Lack of cyclodiene cross-resistance for afoxolaner suggests that afoxolaner blocks GABA-gated chloride channels by binding at a site topologically distinct from the cyclodienes.
Metabolism
Afoxolaner is an isoxazoline compound. It works by blocking the transmissions of neuronal signals in insects. They do this by binding to chloride channels in nerve and muscle cells, causing the receptors to stop working. This in turn paralyses the parasites and kills them.Afoxolaner toxicity to fleas ensures that they die within 24 hours of it being administered. But the dog is unaffected, due to the specific selective nature of the drug.
Mode of action
Afoxolaner (the active ingredient of NEXGARD) and other isoxazolines with insecticidal and tickicidal efficacy are non-competitive GABA (gamma-aminobutyric acid) receptor antagonists, much more selective for GABA receptors in insects or ticks, than for those in mammals, including humans. They bind to chloride channels in nerve and muscle cells, which blocks the transmission of neuronal signals. Affected parasites are paralyzed and die.This mechanism exists not only in insects but also in mammals and other vertebrates. However the binding affinity of afoxolaner to GABA receptors of invertebrates is much higher than to GABA receptors in vertebrates. For this reason it is significantly less toxic to mammals than to insects and other pests.
References
- Efficacy of afoxolaner (NexGard?) against natural infestations with Trichodectes canis in dogs under field conditions DOI:10.1186/s13071-022-05428-y
- Safety of oral afoxolaner formulated with or without milbemycin oxime in homozygous MDR1‐deficient collie dogs DOI:10.1111/jvp.13064
- https://www.ema.europa.eu/en/documents/product-information/nexgard-epar-product-information_en.pdf
- MADISON BIOMEDICAL TECH CHENGDU - CN112457267, 2021, A
-
The Inhibitor Index: A Desk Reference on Enzyme Inhibitors, Receptor Antagonists, Drugs, Toxins, Poisons, Biologics, and Therapeutic Leads DOI:10.1201/9781315184289
- https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a668d223-47a3-46b7-b642-f2de0dca6ab5
Properties of afoxolaner
| Density | 1.53±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO: ≥ 250 mg/mL (399.44 mM) |
| pka | 12.45±0.46(Predicted) |
| form | A crystalline solid |
| color | White to off-white |
Safety information for afoxolaner
Computed Descriptors for afoxolaner
| InChIKey | OXDDDHGGRFRLEE-UHFFFAOYSA-N |
| SMILES | C1(C(NCC(=O)NCC(F)(F)F)=O)=C2C(C=CC=C2)=C(C2CC(C3=CC(C(F)(F)F)=CC(Cl)=C3)(C(F)(F)F)ON=2)C=C1 |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 093861-60-9 AfoxolanerView Details
093861-60-9 AfoxolanerView Details
093861-60-9 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1