Flunarizine
- CAS NO.:52468-60-7
- Empirical Formula: C26H26F2N2
- Molecular Weight: 404.49
- MDL number: MFCD00242731
- EINECS: 257-937-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-10 09:00:29
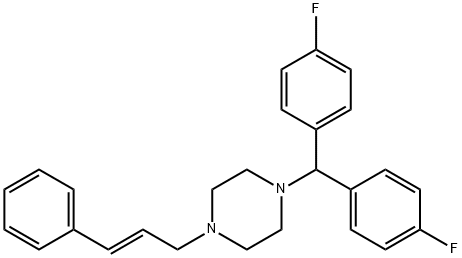
What is Flunarizine?
Absorption
85% following oral administration.
Toxicity
-Flunarizine should be used with care in patients with depression or those being prescribed other agents, such as phenothiazines, concurrently, which may cause extrapyramidal side-effects. -Acute overdosage has been reported and the observed symptoms were sedation, agitation and tachycardia. -Treatment of acute overdosage consists of charcoal administration, induction of emesis or gastric lavage, and supportive measures. No specific antidote is known.
Description
Flunarizine is the difluorinated derivative of cinnarizine. Thus, its preparation and therapeutic use are identical to those of cinnarizine.
Originator
Sibelium,Janssen,W. Germany,1977
The Uses of Flunarizine
Vasodilator.
Indications
Used in the prophylaxis of migraine, occlusive peripheral vascular disease, vertigo of central and peripheral origin, and as an adjuvant in the therapy of epilepsy.
Background
Flunarizine is a selective calcium entry blocker with calmodulin binding properties and histamine H1 blocking activity. It is effective in the prophylaxis of migraine, occlusive peripheral vascular disease, vertigo of central and peripheral origin, and as an adjuvant in the therapy of epilepsy.
Definition
ChEBI: Flunarizine is a diarylmethane.
Manufacturing Process
A mixture of 14.3 parts of di-(p-fluorophenyl)-chloromethane, 10.1 parts of 1-
cinnamylpiperazine, 12.7 parts of sodium carbonate, a few crystals of
potassium iodide in 200 parts of 4-methyl-2-pentanone is stirred and refluxed
for 21 hours. The reaction mixture is cooled and 50 parts of water are added.
The organic layer is separated, dried, filtered and evaporated.
The oily residue is dissolved in 480 parts of anhydrous diisopropyl ether. This
solution is boiled with activated charcoal, filtered and to the clear filtrate is
added an excess of 2-propanol, previously saturated with gaseous hydrogen
chloride. The precipitated salt is filtered off and recrystallized from a mixture
of 2-propanol and ethanol, yielding 1-cinnamyl-4-(di-p-fluorobenzhydryl)
piperazine dihydrochloride, MP 251.5°C.
Therapeutic Function
Vasodilator
World Health Organization (WHO)
Flunarizine, an antihistaminic and vasodilator agent, was introduced into medicine in 1970. It is indicated for the treatment of central and peripheral vascular disorders. However, its effectiveness in these conditions has not been convincingly demonstrated, and its use has been associated with adverse reactions involving the central nervous system, including extrapyramidal disturbances and depression. This has led several regulatory authorities to restrict the approved indications for products containing flunarizine.
Pharmacokinetics
Flunarizine is a selective calcium entry blocker with calmodulin binding properties and histamine H1 blocking activity.
Metabolism
Hepatic, to two metabolites via N-dealylation and hydroxylation.
Properties of Flunarizine
| Boiling point: | 511.3±50.0 °C(Predicted) |
| Density | 1.170±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| pka | 6.99±0.10(Predicted) |
| form | Solid |
| color | White to off-white |
| CAS DataBase Reference | 52468-60-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Piperazine, 1-[bis(4-fluorophenyl)methyl]-4-(3-phenyl-2-propenyl)-, (e)-(52468-60-7) |
Safety information for Flunarizine
Computed Descriptors for Flunarizine
Flunarizine manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran





You may like
-
 52468-60-7 98%View Details
52468-60-7 98%View Details
52468-60-7 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
