fluclorolone acetonide
- CAS NO.:3693-39-8
- Empirical Formula: C24H29Cl2FO5
- Molecular Weight: 487.392
- MDL number: MFCD00200362
- EINECS: 223-010-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:38
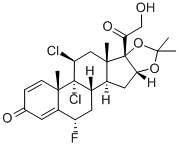
What is fluclorolone acetonide?
Originator
Topilar,Syntex,UK,1971
The Uses of fluclorolone acetonide
fluclorolone acetonide is a synthetic glucocorticoid with anti-inflammatory properties. Studies suggest that it is a potent inhibitor of mouse skin tumor promotion and epidermal DNA synthesis.
Background
Fluclorolone acetonide (INN) or flucloronide (USAN) is a topical corticosteroid. It is marketed under the brand names Cutanit and Topicon.
Definition
ChEBI: Fluclorolone acetonide is a 21-hydroxy steroid.
Manufacturing Process
To 6α-fluoro-16α-hydroxy-hydrocortisone 21-acetate, described by Mills et al,
J. Am. Chem. Soc., volume 81, pages 1264 to 1265, March 5, 1959, there
was added acetic anhydride in dry pyridine. The reaction mixture was left at
room temperature overnight and was then poured with stirring into ice water.
The resulting precipitate was filtered, washed with water and crystallized from
acetone-hexane to give 6α-fluoro-16α-hydroxy-hydrocortisone-16α,21-
diacetate. This was reacted with methane-sulfonyl chloride in dimethyl
formamide in the presence of pyridine at 80°C for 1 hour. The mixture was
cooled, diluted with water and extracted with ethyl acetate. The extract was
washed with water, dried over anhydrous sodium sulfate and the ethyl acetate
was evaporated. By recrystallization of the residue from acetone-hexane there
was obtained 6α-fluoro-Δ4,9(11)-pregnadiene-16α,17α,21-triol-3,20-dione-
16α,21-diacetate.
This was reacted with chlorine to give the dichloropregnene compound, then
with selenium dioxide to give the dichloropregnadiene compound. By
hydrolysis with methanolic potassium hydroxide there was obtained the free
6α-fluoro-9α,11β-dichloro-Δ1,4-pregnadiene-16α,17α,21-triol-3,20-dione. By
treatment with acetone in the presence of perchloric acid, the 16,17-acetonide
of 6α-fluoro-9α,11β-dichloro-Δ1,4-pregnadiene-16α,17α,21-triol-3,20-dione
was formed.
Therapeutic Function
Glucocorticoid
Metabolism
Not Available
Properties of fluclorolone acetonide
| Boiling point: | 598.3±50.0 °C(Predicted) |
| Density | 1.38±0.1 g/cm3(Predicted) |
| pka | 12.87±0.10(Predicted) |
Safety information for fluclorolone acetonide
Computed Descriptors for fluclorolone acetonide
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4