fluazacort
- CAS NO.:19888-56-3
- Empirical Formula: C25H30FNO6
- Molecular Weight: 459.51
- EINECS: 243-400-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 23:16:16
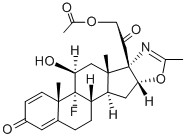
What is fluazacort?
Originator
Azacortid,Richter,Italy,1975
The Uses of fluazacort
fluazacort is a glucucorticoid with anti-inflammatory properties used in the treatment of skin diseases.
Definition
ChEBI: Fluazacort is a corticosteroid hormone.
Manufacturing Process
To a solution of 2.4 g of pregna-1,4,9(11)-triene-21-ol-3,20-dione-[17α,16α-
d]-2'-methyloxazoline 21-acetate in 24 ml of tetrahydrofuran, 12.8 ml of 0.46
N perchloric acid are added at 15°C under stirring. N-bromoacetamide (1.1 g)
is then added to the mixture which is kept far from light, and stirred for 4
hours at room temperature. After lowering the temperature to 10°C, a
saturated solution of sodium bisulfite is added in order to decolorize the
mixture, which is then poured into 120 ml of ice water. A product separates,
which is collected by filtration, washed with water and then dried, thus
obtaining 2.81 g of crude 9α-bromo-pregna-1,4-diene-11β,21-diol-3,20-dione-
[17α,16α-d]-2'-methyloxazoline-21-acetate (yield 93%), MP 175°C to 176°C.
An amount of 2.75 g of 9α-brorno-pregna-1,4-diene-11β,21-diol-3,20-dione-
[17α,16α-d]-2'-methyloxazoline-21-acetate is dissolved under nitrogen in 137
ml of a mixture methanol:chloroform (3:2). The solution is put in ice bath and
5.5 ml of 1 N NaOH are then added within 10 minutes followed by 5.5 ml
within the next 40 minutes. A strong stirring is provided for 2 hours and the
temperature is kept between 0°C and 5°C, then the pH is adjusted to 7 to 8
with glacial acetic acid. The solvent is evaporated in vacuo to 20 ml of volume
of solution, that is poured into ice water (130 ml). The product is collected by
filtration, washed with water and dried. Yield: 1.6 g (80%), MP 221°C to
222°C. It is pregna-1,4-diene-9β,11β-epoxy-21-ol-3,20-dione-[17α,16α-d]-2'-
methyloxazoline.
An amount of 1 g of the above product is dissolved in 9.4 ml of a mixture
obtained by mixing 4.67 ml of hydrofluoric acid with 8.5 ml of tetrahydrofuran
at the temperature of 0°C. This solution is stirred for 20 hours at the same
temperature, then under strong stirring and cooling 20 ml of tetrahydrofuran
are added. The solution is subsequently neutralized by the addition of 24 g of
sodium bicarbonate followed by 1 g of sodium sulfate. The inorganic
substance is collected and washed with ethyl acetate. The filtrate is
evaporated to dryness and the product is crystallized from acetone: 0.65 g
(yield 61%) of pregna-1,4-dien-9α-fluoro-11β,21-diol-3,20-dione-[17α,16α-d]-
2'-methyloxazoline are obtained, MP 241°C to 244°C [α]D = +83.5 (c. 0.5,
CHCl3).The 21-acetate has MP 252°C to 255°C [α]D = +54.8 (c. 0.5, CHCl3).
Therapeutic Function
Antiinflammatory
Properties of fluazacort
| Melting point: | 252-255° |
| alpha | D +54.8° (c = 0.5 in CHCl3) |
Safety information for fluazacort
Computed Descriptors for fluazacort
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1