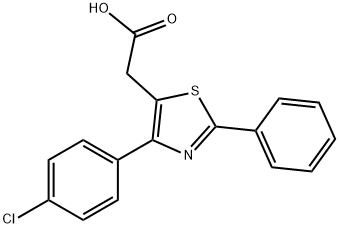
FENTIAZAC
- CAS NO.:18046-21-4
- Empirical Formula: C17H12ClNO2S
- Molecular Weight: 329.8
- MDL number: MFCD00866039
- EINECS: 241-958-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-02 08:55:37
What is FENTIAZAC?
Originator
Norvedan,LPB,Italy,1975
The Uses of FENTIAZAC
Anti-inflammatory.
Definition
ChEBI: 2-[4-(4-chlorophenyl)-2-phenyl-5-thiazolyl]acetic acid is a member of thiazoles.
Manufacturing Process
13.6 g methyl 3-(p-chlorobenzoyl)-3-bromopropionate in 30 ml methanol are
added to a solution of 5.6 g potassium thioacetate in 30 ml methanol.
Immediate precipitation of KBr is observed. The suspension is refluxed for 10
minutes.
It is cooled to ambient temperature, filtered, and the methanol is evaporated
to dryness. 13.2 g methyl 3-(p-chlorobenzoyl)-3-thioacetylpropionate in the
form of a chromatographically pure orange-colored oil are obtained.
A suspension of 13.2 g methyl 3-(p-chlorobenzoyl)-3-thioacetylpropionate is
agitated in 500 ml of a 2 N aqueous solution of KOH for 6 hours at ambient
temperature in an atmosphere of nitrogen, followed by extraction with ethyl
ether. The aqueous phase, adjusted to a pH equal to 2 with 2N HCl, is
extracted with ethyl ether which was washed with water, dried over Na2SO4,
and finally evaporated to dryness
9.8 g of crude 3-(p-chlorobenzoyl)-3-mercaptopropionic acid are obtained. By
recrystallizing from isopropyl ether there are obtained 8.6 g of pure product,
MP 96°C to 97°C (yield: 79%).
1.7 ml benzonitrile and 5.05 ml diethylamine are added to a solution of 4 g 3-
(p-chlorobenzoyl)-3-thiol-propionic acid in 50 ml ethanol. The solution is
agitated at ambient temperature for 60 minutes in an atmosphere of nitrogen.
It is then evaporated to a syrupy consistency and 60 ml 50% aqueous acetic
acid are added, whereupon the mixture is refluxed for 60 minutes. It is
evaporated to a small volume, adjusted to a pH equal to 8 with a saturated
solution of sodium bicarbonate and then extracted with ethyl ether. The
aqueous phase is acidified with 2N HCl (Congo red), and then again extracted
with ethyl ether. It is dried over Na2SO4 and evaporated to dryness. The
evaporation residue is recrystallized from benzene and 4 g 4-(p-chlorophenyl)-
2-phenyl-thiazol-5-yl-acetic acid are obtained (MP = 152°C to 154°C, yield -
74.3%).
Therapeutic Function
Analgesic, Antipyretic, Antiinflammatory
General Description
Fentiazac is reported to have anti-inflammatory, analgesic, and antipyretic activity. It has been given once or twice a day at levels between 100 and 200 mg/dose in the treatment of postoperative pain, including that following orthopedic surgery. The most common adverse effect is gastrointestinal intolerance, including epigastric pain, nausea, and vomiting. Effects on the CNS, such as headache and dizziness, also have been reported.
Trade name
Atilan (Zambon, Brazil), Donorest (Fontoura-Wyeth, Brazil), Flogene (Polifarma, Italy), Norvedan (Boehringer Mannheim, Austria; LPB, Italy).
Synthesis
Synthesis: refluxing a mixture of 3-bromo-
3-(4-chlorobenzoyl)propionic acid and thiobenzamide in ethanol gives ethyl 2-phenyl-4-(4-
chlorophenyl)thiazole-5-acetate, which is then
saponified to yield fentiazac.
Properties of FENTIAZAC
| Melting point: | 162°C |
| Boiling point: | 556.2±60.0 °C(Predicted) |
| Density | 1.1592 (rough estimate) |
| refractive index | 1.6100 (estimate) |
| pka | 3.89±0.10(Predicted) |
| form | Solid |
| color | White to off-white |
| Water Solubility | 31.66mg/L(25 ºC) |
Safety information for FENTIAZAC
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for FENTIAZAC
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION INOSITOL PANTOPRAZOLE SODIUM SESQUIHYDRATE Metformin FLUCLOXACILLIN SODIUM STERILE NIMESULIDE BP ZONISAMIDE 1,1-Dimethylethyl 4-(2-azidoacetyl)-1-piperidinecarboxylate 2-Bromo-1-(bromomethyl)-3-iodo-5-nitrobenzene 1,2-Dibromo-3-(bromomethyl)-5-nitrobenzene 2-Cyano-5-pyrimidineacetonitrile 7-Fluoro-4-cinnolinamine 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzeneRelated products of tetrahydrofuran
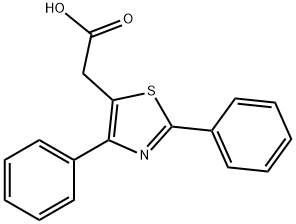
![4-(4-CHLOROPHENYL)-2-METHYL-1,3-THIAZOL-5-YL]ACETIC ACID](https://img.chemicalbook.in/CAS/GIF/553630-41-4.gif)
![METHYL 2-[4-(4-CHLOROPHENYL)-2-PHENYL-1,3-THIAZOL-5-YL]ACETATE](https://img.chemicalbook.in/StructureFile/ChemBookStructure8/GIF/CB9133431.gif)

You may like
-
 1823754-06-8 98%View Details
1823754-06-8 98%View Details
1823754-06-8 -
 2250220-37-0 4-azido-4-methylchromane 98%View Details
2250220-37-0 4-azido-4-methylchromane 98%View Details
2250220-37-0 -
 1935373-20-8 3-(Bromomethyl)-6-fluoro-1,2-benzenediamine 98%View Details
1935373-20-8 3-(Bromomethyl)-6-fluoro-1,2-benzenediamine 98%View Details
1935373-20-8 -
 2-Chloro-5-(iodomethyl)pyrimidine 2268818-88-6 98%View Details
2-Chloro-5-(iodomethyl)pyrimidine 2268818-88-6 98%View Details
2268818-88-6 -
 2092793-98-9 98%View Details
2092793-98-9 98%View Details
2092793-98-9 -
 1360944-55-3 7-Methoxy-1H-indol-5-amine 98%View Details
1360944-55-3 7-Methoxy-1H-indol-5-amine 98%View Details
1360944-55-3 -
 2090480-14-9 98%View Details
2090480-14-9 98%View Details
2090480-14-9 -
![1-Methyl 2-bromo-5-[(1-carboxy-1-methylethyl)amino]benzoate 1651844-56-2 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-Methyl 2-bromo-5-[(1-carboxy-1-methylethyl)amino]benzoate 1651844-56-2 98%View Details
1-Methyl 2-bromo-5-[(1-carboxy-1-methylethyl)amino]benzoate 1651844-56-2 98%View Details
1651844-56-2