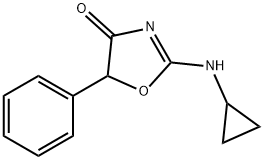
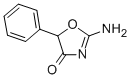
fenozolone
- CAS NO.:15302-16-6
- Empirical Formula: C11H12N2O2
- Molecular Weight: 204.23
- MDL number: MFCD00868084
- EINECS: 2393396
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-15 10:43:56
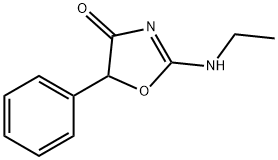
What is fenozolone?
Originator
Fenozolone,Shanghai Lansheng
The Uses of fenozolone
Fenozolone is a psychoactive drug and stimulant related to pemoline and 4-methylaminorex which acts as a norepinephrine-dopamine releasing agent (NDRA).
Definition
ChEBI: Fenozolone is a member of benzenes.
Manufacturing Process
Into a 3-necked spherical flask of 1 L provided with a dropping funnel, a condenser surmounted by a calcium chloride tube and a mechanical stirrer, is introduced a suspension of 17.6 g (0.2 mol) of ethyl urea in 150 ml of anhydrous benzene. There is added through the dropping funnel in 20 minutes a solution of 18.9 g (0.1 mol) α-chlorophenyl-acetyl chloride in 300 ml of benzene. The mixture is left at ambient temperature for 15 minutes and is then heated under reflux on the water bath with stirring for 5 hours. The benzene solution is decanted at elevated temperature in order to separate it from an oil deposited on the bottom of the flask, the benzene is driven off on the water bath, the last traces removed in vacuum, the crystalline residue is triturated in a mortar in about 200 ml of water, and the crystalline solid is separated off and is with water and dried in vacuum over phosphorus pentoxide. There are thus recovered 19.6 g (yield 82%) of 1-(2-chloro-2- phenyl-acetyl)-3-ethyl-urea, which when recrystallized from 80 ml of benzene, takes the form of white crystals soluble in benzene but insoluble in water. The product has melting point 146°C. To a suspension of 39 g (0.163 mol) of 1-(2-chloro-2-phenyl-acetyl)-3-ethylurea in 250 ml of anhydrous ethyl alcohol is added a sodium ethoxide solution containing 3.75 g (0.163 mol) of sodium dissolved in 250 ml of ethyl alcohol. The mixture is heated under reflux for 2 hours and left overnight at ambient temperature. The precipitated sodium chloride is separated off and copiously washed with alcohol. The alcohol is driven off from the filtrate on the water bath, the oily residue is triturated in 20 ml of iced water, and the solid formed is refrigerated for several hours, separated, washed with water and dried in vacuum over phosphorus pentoxide. The 5-phenyl-2-ethylamino-4-oxazolinone (fenozolone) obtained is recrystallized from anhydrous benzene. It then forms a white crystalline compound soluble in benzene and insoluble in water, MP: 148°C.
Therapeutic Function
Psychostimulant
Properties of fenozolone
| Melting point: | 148° |
| Boiling point: | 306.8±45.0 °C(Predicted) |
| Density | 1.23±0.1 g/cm3(Predicted) |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 1.83±0.40(Predicted) |
| color | White to Off-White |
Safety information for fenozolone
Computed Descriptors for fenozolone
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 6-Bromo-3-iodo-1-methyl-1H-indazole N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine N-Boc-L-proline methyl ester 2-(BOC-Amino)4-picoline 1-(4-Methylphenylsulfonyl)-1H-1,2,3-benzotriazole 1-(2-Chlorobenzyl)-4-nitro-1H-pyrazoleRelated products of tetrahydrofuran
You may like
-
 55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 -
 181228-33-1 99%View Details
181228-33-1 99%View Details
181228-33-1 -
 Ste-Glu-AEEA-AEEA-OSUView Details
Ste-Glu-AEEA-AEEA-OSUView Details
1169630-40-3 -
 1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 -
 127464-43-1 99%View Details
127464-43-1 99%View Details
127464-43-1 -
 Chloro Uracil 99%View Details
Chloro Uracil 99%View Details
1820-81-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 13162-05-5 99%View Details
13162-05-5 99%View Details
13162-05-5