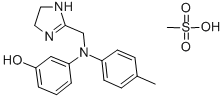
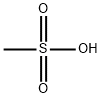
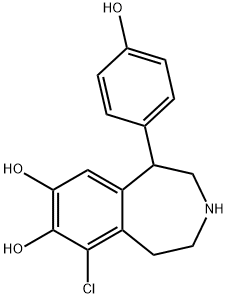
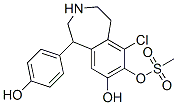
Fenoldopam mesylate
Synonym(s):Fenoldopam methanesulfonate
- CAS NO.:67227-57-0
- Empirical Formula: C17H20ClNO6S
- Molecular Weight: 401.86
- MDL number: MFCD04112986
- EINECS: 266-612-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-08 09:02:20
What is Fenoldopam mesylate?
Description
Fenoldopam, first approved in the Netherlands in 1992, ended by reaching its first market, the US, for the short-term management of severe hypertension, including malignant hypertension, in the hospital setting. Fenoldopam can be prepared in 3 steps from the corresponding phenethylamine and aryloxiran, the pivotal step being the cyclisation in benzazepine in acidic medium. Fenoldopam is a potent dopamine D1 receptor agonist acting peripherally to produce systemic vasodilation.As it does not cross the bloodbrain barrier, it does not exert significant central dopaminergic activity. Fenoldopam also interacts significantly with 5HT1c and 5HT2 receptors. In comparative trials with the most common drug used for this condition in Europe, Fenoldopam was found to be appreciably more potent than nifedipine. Furthermore, Fenoldopam is fast acting and maintains a long-lasting antihypertensive effect.
Description
Fenoldopam is an agonist of dopamine D1A (D1R) and D1B (D5R) receptors (Kds = 17 and 11 nM, respectively). Fenoldopam is used to study the roles of these receptors, in cells and in vivo, and to alter hemodynamic properties, including hypertension, in animals.
Chemical properties
White to Off-White Solid
Originator
SmithKline Beecham (US)
The Uses of Fenoldopam mesylate
Fenoldopam mesylate may be used to study dopamine D1-mediated cell signaling.
The Uses of Fenoldopam mesylate
Dopamine D1-receptor agonist. Antihypertensive.
The Uses of Fenoldopam mesylate
antihypertensive, dopamine agonist
What are the applications of Application
Fenoldopam Mesylate is an anti-hypertensive, dopamine D1-receptor agonist
Definition
ChEBI: Fenoldopam mesylate is a benzazepine.
Manufacturing Process
2-Chloro-3,4-dimethoxyphenethylamine (1.0 g) was reacted with 0.70 g of pmethoxystyrene
oxide to give the hydroxyphenethylamine; m.p. 118.5-121°C.
This compound (2.16 g) was stirred at room temperature in 15 ml of
trifluoroacetic acid with 4 drops of conc. sulfuric acid. After purification over a
silica gel column with chloroform, 10% methanol/chloroform as eluates, was
obtained 6-chloro-7,8-dimethoxy-1-p-methoxyphenyl-2,3,4,5-tetrahydro-1H-
3-benzazepine (0.78 g), m.p. 143-145°C.
The trimethoxy product (0.87 g, 2.50 mmoles) in 25 ml of dry methylene
chloride was cooled in an ice-methanol bath and 12.5 ml (25.0 mmoles) of
boron tribromide in methylene chloride was added dropwise. After stirring for
4 hours, the mixture was cooled in an ice bath while methanol was carefully
added to give 0.37 g of 6-chloro-7,8-dihydroxy-1-p-hydroxyphenyl-2,3,4,5-
tetrahydro-1H-3-benzazepine hydrobromide, m.p. 215°C.
The base was regenerated from the hydrobromide salt using sodium carbonate solution in 85% yield. Treating the base with various acids gave the
following salts: dl-tartrate, fumarate, hydrochloride, sulfate, and the most
water soluble one, the methanesulfonate, m.p. 272°C.
brand name
Corlopam (Hospira).
Therapeutic Function
Antihypertensive
Biochem/physiol Actions
Fenoldopam mesylate (FM) is considered as an effective therapeutic agent to prevent the onset of postoperative AKI (PO-AKI). In healthy cats, FM can stimulate diuresis and be well-tolerated. This benzazepine-derivative is regional specific and does not cause cerebral vasodilation.
Properties of Fenoldopam mesylate
| Melting point: | 274° (dec) |
| storage temp. | room temp |
| solubility | DMSO: ≥15mg/mL at ~60°C |
| form | powder |
| color | white to tan |
| CAS DataBase Reference | 67227-57-0(CAS DataBase Reference) |
Safety information for Fenoldopam mesylate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Fenoldopam mesylate
| InChIKey | WOFIIEUMIDZJEJ-UHFFFAOYSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Fenoldopam methanesulfonate 98% CAS 67227-57-0View Details
Fenoldopam methanesulfonate 98% CAS 67227-57-0View Details
67227-57-0 -
 Fenoldopam methanesulfonate 95.00% CAS 67227-57-0View Details
Fenoldopam methanesulfonate 95.00% CAS 67227-57-0View Details
67227-57-0 -
 Fenoldopam mesylate CAS 67227-57-0View Details
Fenoldopam mesylate CAS 67227-57-0View Details
67227-57-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1