Fenfluramine hydrochloride
- CAS NO.:404-82-0
- Empirical Formula: C12H17ClF3N
- Molecular Weight: 267.72
- MDL number: MFCD00058016
- EINECS: 206-968-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-07-09 08:28:05
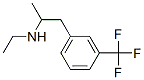
What is Fenfluramine hydrochloride?
Chemical properties
Crystalline Solid
The Uses of Fenfluramine hydrochloride
Controlled substance. Anorexic
Definition
ChEBI: The hydrochloride salt of fenfluramine. It binds to the serotonin reuptake pump, causing inhbition of serotonin uptake and release of serotonin. The resulting increased levels of serotonin lead to greater serotonin receptor activation which in turn lead to enhancement of serotoninergic transmission in the centres of feeding behavior located in the hypothalamus. This suppresses the appetite for carbohydrates. Fenfluramine hydrochloride was used for treatment of diabetes and obesity. It was withdrawn worldwide after reports of heart valve disease and pulmonary hypertension.
brand name
Pondimin (Robins).
General Description
Fenfluramine hydrochloride, (±)N-ethyl- -methyl-m-(trifluoromethyl)phenethylamine hydrochloride (Pondimin), isunique in this group of drugs, in that it tends to produce sedationrather than excitation. Effects are said to be mediatedprincipally by central serotoninergic, rather than central noradrenergic,mechanisms. In large doses in experimental animals,the drug is a serotonin neurotoxin.It was withdrawnfrom human use after reports of heart valve damage and pulmonaryhypertension. From its structure, more apolar or hydrophobiccharacter than amphetamine, tropism for serotoninergicneurons would be expected. Likewise, thestructure suggests an indirect mechanism. If an indirectmechanism were operative, then all postsynaptic 5-HT receptors could be activated. Evidence from several studies indicatesthat the 5-HT1B and the 5-HT2C receptors are mostresponsible for the satiety effects of 5-HT. 5-HT may alsoinfluence the type of food selected (e.g., lower-fat food intake).The(+) isomer, dexfenfluramine (Redux), has agreater tropism for 5-HT systems than the racemic mixture.It, too, was withdrawn because of toxicity.
Safety Profile
Poison by ingestion, intravenous, and intraperitoneal routes. Human systemic effects by ingestion: mydriasis, change in motor activity, nausea. An experimental teratogen. Other experimental reproductive effects. When heated to decomposition it emits very toxic fumes of F-, NOx, and HCl
Properties of Fenfluramine hydrochloride
| Melting point: | 1660C |
| Boiling point: | 108-112 °C(Press: 12 Torr) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Sparingly) |
| form | A solid |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 404-82-0(CAS DataBase Reference) |
Safety information for Fenfluramine hydrochloride
Computed Descriptors for Fenfluramine hydrochloride
Fenfluramine hydrochloride manufacturer
Biophore India Pharmaceuticals Pvt Ltd
VCS Chem Tech Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 404-82-0 Fenfluramine hydrochloride 98%View Details
404-82-0 Fenfluramine hydrochloride 98%View Details
404-82-0 -
 404-82-0 Fenfluramine hydrochloride 98%View Details
404-82-0 Fenfluramine hydrochloride 98%View Details
404-82-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1