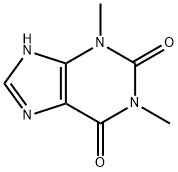
fenetylline hydrochloride
- CAS NO.:1892-80-4
- Empirical Formula: C18H24ClN5O2
- Molecular Weight: 377.86846
- EINECS: 217-580-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-29 13:57:48
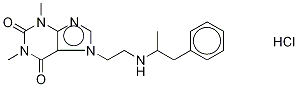
What is fenetylline hydrochloride?
Description
Fenethylline is a combination of the drugs amphetamine and theophylline. It was formerly used to treat conditions such as ADHD, narcolepsy, and depression; but its use has been banned because of the potential for abuse.
Chemists at the German drug company Degussa AG first synthesized fenethylline in 1961 from amphetamine and 7-(2-chloroethyl)theophylline. The company soon began to market its hydrochloride salt (shown) under the trade name Captagon. Its sales continued until 1986, when most countries outlawed it.
Fenethylline re-emerged this year because of its widespread abuse by Middle Eastern young adults. It is promoted by terrorist groups such as the Islamic State to enhance what they consider to be desirable characteristics—aggressiveness, alertness, and fearlessness—in their recruits.
In the body, enzymes oxidize fenethylline to produce its original components, amphetamine and theophylline, each of which has its own undesirable characteristics when taken in large quantities. To counteract these effects, Cody J. Wenthur, Bin Zhou, and Kim D. Janda* at the Scripps Research Institute (La Jolla, CA) developed?protein-conjugate vaccines that disarm fenethylline and its constituents’ abilities to affect brain activity. ?
The authors’ next objective is to develop a combination vaccine for treating fenethylline addiction.
Originator
Captagon,Homburg,W. Germany,1961
The Uses of fenetylline hydrochloride
CNS stimulant. Controlled substance.
Manufacturing Process
1 mol of 7-(β-chloroethyl)-theophylline and 2? mols of α-methyl-β-phenyl
ethylamine are heated for 6 hours in an oil bath, if necessary with addition of
alcohol or toluene. The reaction mixture is diluted with alcohol and acidified
with alcoholic hydrochloric acid. The crystalline mass formed is filtered with
suction and extracted by boiling with alcohol. A product having a melting point
of 237°C to 239°C is formed. With prolonged extraction by boiling with
alcohol, the melting point of the mass falls, preferably due to a change in
modification, to 227°C to 229°C. However, analysis shows that both products
are the pure condensation product.
Instead of the chloroethyl theophylline, it is also possible to use the
corresponding bromine derivative. It was found that in this way the process is
facilitated and the yield is improved
Therapeutic Function
Central stimulant
Properties of fenetylline hydrochloride
| Melting point: | 227-229°; mp 237-239° |
| solubility | 127 mg/l |
| solubility | DMF: 0.3 mg/ml; DMSO: 0.5 mg/ml; PBS (pH 7.2): 10 mg/ml |
| form | A crystalline solid |
| appearance | white crystalline powder |
Safety information for fenetylline hydrochloride
Computed Descriptors for fenetylline hydrochloride
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8