43210-67-9
Product Name:
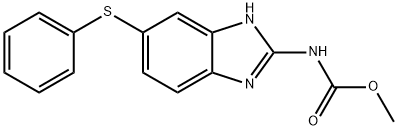
Fenbendazole
Formula:
C15H13N3O2S
Synonyms:
Fenbendazole;Fenbendazole Impurity B (PhEur);Methyl (5-chloro-1H-benzimidazol-2-yl)carbamate;Methyl 5-(phenylthio)-2-benzimidazolecarbamate
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Melting Point | 233 °C |
| Solubility | 0.9 [ug/mL] (The mean of the results at pH 7.4) |
| Collision Cross Section | 170.8 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards] |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 299.3 g/mol |
|---|---|
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 299.07284784 g/mol |
| Monoisotopic Mass | 299.07284784 g/mol |
| Topological Polar Surface Area | 92.3 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 363 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Fenbendazole is a broad-spectrum anthelmintic drug that is particularly used in veterinary medicine for the treatment of nematodal infections. It is a member of the benzimidazole class, which is a type of compound that has a 1H-benzimidazole core. This core is substituted at positions 2 and 5 with specific functional groups: (methoxycarbonyl)amino and phenylsulfanediyl groups, respectively. Fenbendazole also has roles as an antinematodal drug and is classified as a carbamate ester and an aryl sulfide.
