FADROZOLE HYDROCHLORIDE
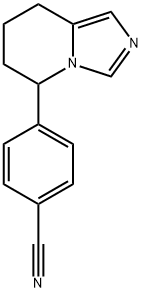
Synonym(s):4-(5,6,7,8-Tetrahydroimidazo[1,5-a]pyridin-5-yl)-benzonitrile;Afema;CGS 16949A
- CAS NO.:102676-31-3
- Empirical Formula: C14H13N3.ClH
- Molecular Weight: 259.739
- MDL number: MFCD00866239
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-15 14:23:52
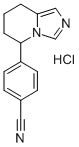
What is FADROZOLE HYDROCHLORIDE?
Description
Fadrozole hydrochloride is a non-steroidal imidazole derivative launched for the treatment of post-menopausal breast cancer. Fadrozole is a potent and specific aromatase inhibitor with neither androgenic nor estrogenic activities due to its structural novelty. Fadrozole exerts its action by coordinating with the iron of the porphyrin nucleus, presumably through the imidazole moiety. This strong binding competes with the binding of molecular oxygen to iron and reversibly inactivates the enzyme. An excellent clinical response rate has been reported for fadrozole with no significant side effects.
Originator
Ciba-Geigy (Switzerland)
The Uses of FADROZOLE HYDROCHLORIDE
Fadrozole hydrochloride is a very potent and selective inhibitory effect of the aromatase enzyme system.
The Uses of FADROZOLE HYDROCHLORIDE
Antineoplastic.
What are the applications of Application
Fadrozole hydrochloride is a very potent and selective inhibitory effect of the aromatase enzyme system
Definition
ChEBI: Fadrozole hydrochloride is an imidazopyridine.
Manufacturing Process
5-p-Cyanophenyl-5,6,7,8-tetrahydroimidazo[1,5-a]pyridine hydrochloride:
A solution of 2.0 g of 4-(4-chloro-4-p-cyanophenyl-n-butyl)-1H-imidazole in
50 ml of chloroform is refluxed for 4 hours under nitrogen, cooled and
evaporated to yield the 5-p-cyanophenyl-5,6,7,8-tetrahydroimidazo[1,5-a]pyridine hydrochloride; melting point 231-233°C (from 2-propanol).
Preparation of the starting materials:
A solution of 1.82 g of 4-(3-ethoxycarbonylpropyl)-1H-imidazole in 30 ml of
tetrahydrofuran under nitrogen is treated with 0.5 g of sodium hydride (50%
oil dispersion) at 0°C for 30 min and 1.45 ml of trimethylsilyl chloride at 0°C
for 3 hours. The reaction mixture is washed with cold 0.5 N sodium
bicarbonate solution, dried over sodium sulfate and evaporated to dryness.
The oil is redissolved in 100 ml of methylene chloride at -78°C under nitrogen
and 12.82 ml of diisobutylaluminum hydride (1.56 M) is added dropwise. The
reaction mixture is stirred for 5 min at -78°C, quenched with 1 ml of
methanol followed by 10 ml of water and filtered through Celite?. The organic
phase is separated, dried over sodium sulfate and evaporated to yield the title
compound (a).
(b) 4-(4-p-t-Butylaminocarbonylphenyl-4-hydroxy-n-butyl)-1-trimethylsilylim
idazole:6.95 g of p-tert-butylaminocarbonylbromobenzene is dissolved in 175 ml of
tetrahydrofuran at -70°C under nitrogen and 20.1 ml of a solution of n-butyl
lithium (2.7 m) in hexane is added dropwise. After reacting 30 min, a solution
of 5.69 g of 4-(3-formyl-n-propyl)-1-trimethylsilyl imidazole in 10 ml of
tetrahydrofuran is added slowly. The reaction mixture is allowed to warm
slowly to room temperature and 20 ml of ammonium chloride is added. The
organic layer is separated, dried over sodium sulfate and evaporated to yield
the title compound (b).
(c) 4-(4-Chloro-4-p-cyanophenyl-n-butyl)-1H-imidazole:
A solution of 4.5 g of 4-(4-p-t-butylaminocarbonylphenyl-4-hydroxy-n-butyl)-
1-trimethylsilylimidazole in 50 ml of thionyl chloride is refluxed for 1 hour,
cooled and evaporated. The residue is partitioned between methylene chloride
and aqueous sodium bicarbonate solution. The organic phase is separated,
dried over sodium sulfate and evaporated to yield the title compound (c).
5-p-Cyanophenyl-5,6,7,8-tetrahydroimidazo[1,5-a]pyridine:
A solution of 2.0 g of 4-(4-chloro-4-p-cyanophenyl-n-butyl)-1H-imidazole in
50 ml of chloroform is refluxed for 4 hours under nitrogen, cooled and
evaporated to yield the 5-p-cyanophenyl-5,6,7,8-tetrahydroimidazo[1,5-
a]pyridine.
brand name
Afema
Therapeutic Function
Antineoplastic
Biochem/physiol Actions
Fadrozole is a nonsteroidal aromatase inhibitor. Fadrozole is a very potent and highly selective inhibitor of the aromatase enzyme system in vitro and estrogen biosynthesis in vivo. It inhibited the conversion of [4-14C]androstenedione to [4-14C]estrone by human placental microsomes in a competitive manner (Ki = 1.6 nM). At a substrate concentration 3-fold the Km, Fadrozole was 180 times more potent, as an inhibitor, than aminoglutethimide (Cat. No. A9657), exhibiting half-maximal inhibition at 1.7 nM as compared to 0.3 μM. In vivo, Fadrozole lowered ovarian estrogen synthesis by gonadotropin-primed, androstenedione treated, immature rats by 90% at a dose of 260 μg/kg (PO). In vivo, Fadrozole leads to sequelae of estrogen deprivation (e.g. regression of DMBA-induced mammary tumors) without causing adrenal hypertrophy in adult rats. It blocked aromatase by 50% in human breast cancer homogenates, live breast cancer cells, human placental microsomes, and porcine ovarian microsomes at concentrations of 0.008 to 0.02 μM.
Properties of FADROZOLE HYDROCHLORIDE
| Melting point: | 231-233℃ |
| storage temp. | room temp |
| solubility | DMSO: >20mg/mL |
| form | powder |
| color | Pale Beige to Pale Yellow |
| Stability: | Hygroscopic |
Safety information for FADROZOLE HYDROCHLORIDE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H361:Reproductive toxicity |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. |
Computed Descriptors for FADROZOLE HYDROCHLORIDE
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 Fadrozole hydrochloride CAS 102676-31-3View Details
Fadrozole hydrochloride CAS 102676-31-3View Details
102676-31-3 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
