Ethyl vinyl ether
Synonym(s):Ethoxyethylene;Ethyl vinyl ether;Vinyl ethyl ether
- CAS NO.:109-92-2
- Empirical Formula: C4H8O
- Molecular Weight: 72.11
- MDL number: MFCD00009248
- EINECS: 203-718-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
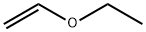
What is Ethyl vinyl ether?
Chemical properties
Colorless liquid. Extremely reactive, can be polymerized in liquid or vapor phase. Slightly soluble in water (0.9% by weight). Commercial material contains inhibitor to prevent premature polymerization. Often stored underground to minimize vapor losses.
The Uses of Ethyl vinyl ether
Ethyl vinyl ether is used in fragrances, lubricating oil additives and spices preparation. It acts as an intermediate for sulfadiazine. It finds application in pharmaceuticals as anesthetics and analgesics. Further, it is used as a solvent in organic synthesis.
The Uses of Ethyl vinyl ether
Copolymerization, intermediate.
Preparation
Ethyl vinyl ether (EVE) can be prepared by reacting acetylene with absolute ethanol in the presence of an alkali catalyst. The most commonly used catalyst for vinylation is an alkali metal hydroxide or an alkali metal alkoxide. 
In China, three processes for the manufacture of EVE using the acetylene route were used:
A continuous process with a homogeneous catalyst under high pressure. The advantages of this process are fast reaction rate and high conversion, but the disadvantages include the requirement of high standard equipment, large energy consumption, and easy safety issues.
A process with a solid catalyst (heterogeneous catalyst) under atmospheric pressure is relatively simple in product separation and refining compared with a process with a homogeneous catalyst under high pressure. However, it has the disadvantages of low output, the short service life of catalyst (about 110 h), and a high requirement on the specification of carrier lime.
A process with a homogeneous catalyst under atmospheric pressure has the advantages of high output and good safety but disadvantages of low conversion.
In the process with solid catalyst under atmospheric pressure, acetylene, and ethanol vapor were introduced into a fixed-bed reactor, and the vinylation reaction was carried out at a temperature as high as about 180 °C. EVE was produced with a concentration of about 70% in the outlet gas stream from the reactor. The fixed-bed reactor was charged with 4–5 mesh catalyst particles of potassium hydroxide supported on lime.
General Description
A clear colorless low-boiling liquid (35-36°C) with an ether-like odor. Flash point below -50°F. May polymerize exothermically if heated or contaminated. If polymerization takes place inside a container, the container may rupture violently. Less dense than water and slightly soluble in water. Hence floats on water. Vapors are heavier than air.
Air & Water Reactions
Highly flammable. Slightly soluble in water. Tends to form explosively unstable peroxides when exposed to oxygen.
Reactivity Profile
Ethyl vinyl ether is a very dangerous fire and explosion hazard when exposed to heat or flame. Undergoes autooxidation with formation of peroxides in the air. Can react vigorously with oxidizing materials. Undergoes explosive polymerization in contact with methanesulfonic acid [Eaton, P. E. et al., J. Org. Chem., 1972, 37, p. 1947].
Hazard
Carcinogen.
Health Hazard
INHALATION OR INGESTION: Excitement followed by unconsciousness and respiratory paralysis. CNS depression. EYES: May cause irritation and transient injury to cornea. SKIN: Prolonged contact can cause tissue defatting and dehydration leading to dermatitis.
Fire Hazard
Behavior in Fire: Explosive hazard
Safety Profile
Mddly toxic by ingestion. Mutation data reported. A skin irritant. A very dangerous fire and explosion hazard when exposed to heat or flame; can react vigorously with oxidizing materials. To fight fire, use alcohol foam, foam, CO2, dry chemical. Explosive polymerization is catalyzed by methane sulfonic acid. When heated to decomposition it emits acrid smoke and irritating fumes. See also ETHERS.
Synthesis
Ethyl vinyl ether is synthesized by bubbling Acetylene through Ethylalcohol in presence of Sodium ethoxide.
Purification Methods
It usually contains polymerization inhibitors (usually amines, e.g. triethanolamine) which can be removed by fractional distillation. Redistil it from sodium. [Beilstein 1 IV 2049.] LACHRYMATORY.
Purification Methods
Ethyl vinyl ether (EVE) mainly contains impurities such as ethanol, acetaldehyde, and acetal and triethanolamine, which are added to prevent hydrolysis and must be refined before use. The purification is done by extensive washing with water or diluting potassium hydroxide solution. Peroxide may be generated in EVE if the latter has been stored for a long time. In this case, potassium iodide dissolved in dioxane can be applied to check if the ether contains peroxide. The peroxide in the ether can be washed by reducing agent solution of ferrous sulfate, sodium sulfite, stannous chloride, and the like, dried by potassium hydroxide and calcium chloride, and finally refined by distillation in the presence of potassium hydroxide or metallic sodium.
Properties of Ethyl vinyl ether
| Melting point: | -116 °C (lit.) |
| Boiling point: | 33 °C (lit.) |
| Density | 0.753 g/mL at 25 °C (lit.) |
| vapor pressure | 560 hPa (20 °C) |
| refractive index | n |
| Flash point: | −50 °F |
| storage temp. | 2-8°C |
| solubility | 8.3g/l |
| form | Liquid |
| color | Clear colorless |
| Odor | nauseating-ethereal but sweet odor |
| explosive limit | 1.3-12.0%(V) |
| Water Solubility | 7.8 g/L (25 ºC) |
| BRN | 605351 |
| Exposure limits | ACGIH: Ceiling 2 mg/m3 NIOSH: Ceiling 2 mg/m3 |
| Stability: | Stable. Highly flammable. Incompatible with strong oxidizing agents. May form peroxides in storage - check for their formation before use. |
| CAS DataBase Reference | 109-92-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Ethene, ethoxy-(109-92-2) |
| EPA Substance Registry System | Vinyl ethyl ether (109-92-2) |
Safety information for Ethyl vinyl ether
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H336:Specific target organ toxicity,single exposure; Narcotic effects H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P242:Use only non-sparking tools. P273:Avoid release to the environment. |
Computed Descriptors for Ethyl vinyl ether
| InChIKey | FJKIXWOMBXYWOQ-UHFFFAOYSA-N |
Ethyl vinyl ether manufacturer
Triveni Interchem Private Limited (Group Of Triveni Chemicals)
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


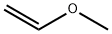

You may like
-
 Ethyl vinyl ether 98%View Details
Ethyl vinyl ether 98%View Details -
 Ethyl vinyl ether 98%View Details
Ethyl vinyl ether 98%View Details
109-92-2 -
 Ethyl vinyl ether, Stabilized CAS 109-92-2View Details
Ethyl vinyl ether, Stabilized CAS 109-92-2View Details
109-92-2 -
 Ethyl vinyl ether, 99% CAS 109-92-2View Details
Ethyl vinyl ether, 99% CAS 109-92-2View Details
109-92-2 -
 Ethyl Vinyl Ether (stabilized with KOH) CAS 109-92-2View Details
Ethyl Vinyl Ether (stabilized with KOH) CAS 109-92-2View Details
109-92-2 -
 Ethyl vinyl ether CAS 109-92-2View Details
Ethyl vinyl ether CAS 109-92-2View Details
109-92-2 -
 Ethyl vinyl ether CAS 109-92-2View Details
Ethyl vinyl ether CAS 109-92-2View Details
109-92-2 -
 Ethyl Vinyl Ether, Purity: 98% Min, IndustrialView Details
Ethyl Vinyl Ether, Purity: 98% Min, IndustrialView Details
109-92-2
