Ethyl methanesulfonate
Synonym(s):Ethyl mesylate;Ethyl methanesulfonate;Methanesulfonic acid ethyl ester
- CAS NO.:62-50-0
- Empirical Formula: C3H8O3S
- Molecular Weight: 124.16
- MDL number: MFCD00007559
- EINECS: 200-536-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
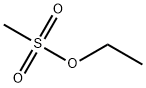
What is Ethyl methanesulfonate?
Description
Ethyl methanesulfonate (EMS), also called ethyl mesylate, is a simple, but hazardous, sulfonic acid ester. Its earliest preparation, reported by German organic chemist O. C. Billeter in 1905, was the reaction of methanesulfonic anhydride with ethyl alcohol.
As shown in the hazard information table, EMS is suspected of being mutagenic, teratogenic, and carcinogenic. In an early mutagenicity study (1965), Thomas Alderson at the University of Cambridge (UK) used EMS to induce mutations in fruit flies (Drosophila spp.).
In 1984, Gary A. Sega at Oak Ridge National Laboratory (TN) published an extensive review of EMS’s genetic effects. And last year, in its 15th Report on Carcinogens, the National Toxicology Program (NTP) of the US Department of Health and Human Services included a data sheet on the carcinogenicity of EMS. In addition, NTP also reported a summary of the testing status of the compound.
Because of its extreme toxicity, EMS is produced only for research purposes. Its primary use is as a model alkylating agent in biochemical and medical research, specifically in studies of DNA repair processes.
Chemical properties
colourless liquid
Chemical properties
Ethyl methane sulfonate is a clear liquid.
The Uses of Ethyl methanesulfonate
Ethyl methanesulfonate is used as mutagen for both mammalian and plant cells. It finds application as a model alkylating agent in the study of deoxyribonucleic acid (DNA) repair processes.
The Uses of Ethyl methanesulfonate
Experimentally as mutagen, teratogen and brain carcinogen.
What are the applications of Application
Ethyl methanesulfonate is a DNA ethylating mutagenic agent
Definition
ChEBI: A methanesulfonate ester resulting from the formal condensation of methanesulfonic acid with ethanol.
General Description
Clear colorless liquid. Denser than water.
Air & Water Reactions
Water soluble.
Reactivity Profile
Ethyl methanesulfonate alkylates nucleophiles such as hydroxy, amino and sulfhydryl groups in model and biological materials. Is hydrolyzed by excess aqueous alkali to non-corrosive and non-toxic products. Is hydrolyzed by water to a highly corrosive product .
Fire Hazard
Ethyl methanesulfonate is combustible.
Biochem/physiol Actions
Ethyl methanesulfonate is a DNA ethylating agent, mutagenic to plants and animals and carcinogenic in mammals. It has been used as a model alkylating agent in studies of DNA repair processes. EMS induces base substitutions of guanine-cytosine (G/C) to adenine-thymine (A/T). EMS also generates point mutations and single nucleotide polymorphisms in genomes. EMS is potential chemical mutagen used for inducing mutation in rice, wheat and Arabidopsis thaliana.
Safety Profile
Confirmed carcinogen with experimental carcinogenic, neoplas tigenic, tumorigenic, and teratogenic data. Poison by ingestion and intraperitoneal routes. Experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits toxic fumes of SOx. See also SULFONATES and ESTERS.
Potential Exposure
Used as a research tool for mutagenesis and carcinogenesis studies. Was considered as a possible human male contraceptive. Also considered as a reversible male hemosterilant for insects and mammalian pests.
Carcinogenicity
Ethyl methanesulfonate is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Shipping
UN2810 Toxic liquids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. Military driver shall be given full and complete information regarding shipment and conditions in case of emergency. AR 50-6 deals specifically with the shipment of chemical agents. Shipments of agent will be escorted in accordance with AR 740-32.
Incompatibilities
Vapors may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides. Contact with moisture may cause hydrolysis or other forms of decomposition
Properties of Ethyl methanesulfonate
| Melting point: | <25 °C |
| Boiling point: | 85-86 °C/10 mmHg (lit.) |
| Density | 1.206 g/mL at 20 °C |
| vapor pressure | 0.27 hPa (25 °C) |
| refractive index | n |
| Flash point: | 100 °C |
| storage temp. | 2-8°C |
| solubility | 50-100g/l |
| form | liquid |
| appearance | colorless liquid |
| color | Colourless to Pale Yellow |
| Water Solubility | Miscible with water. |
| Sensitive | Moisture Sensitive |
| Merck | 14,3827 |
| BRN | 773969 |
| Stability: | Stable. Combustible. Incompatible with water, alkalies, oxidizing agents. |
| CAS DataBase Reference | 62-50-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Methanesulfonic acid, ethyl ester(62-50-0) |
| IARC | 2B (Vol. 7, Sup 7) 1987 |
| EPA Substance Registry System | Ethyl methanesulfonate (62-50-0) |
Safety information for Ethyl methanesulfonate
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H340:Germ cell mutagenicity H350:Carcinogenicity |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Ethyl methanesulfonate
| InChIKey | PLUBXMRUUVWRLT-UHFFFAOYSA-N |
Ethyl methanesulfonate manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 62-50-0 Ethyl methanesulfonate 98%View Details
62-50-0 Ethyl methanesulfonate 98%View Details
62-50-0 -
 Ethyl methanesulfonate 98%View Details
Ethyl methanesulfonate 98%View Details
62-50-0 -
 Ethyl Methanesulphonate (EMS) extrapure CAS 62-50-0View Details
Ethyl Methanesulphonate (EMS) extrapure CAS 62-50-0View Details
62-50-0 -
 Ethyl methane sulphonate, 98% CAS 62-50-0View Details
Ethyl methane sulphonate, 98% CAS 62-50-0View Details
62-50-0 -
 Ethyl Methanesulfonate CAS 62-50-0View Details
Ethyl Methanesulfonate CAS 62-50-0View Details
62-50-0 -
 Ethyl methanesulfonate CAS 62-50-0View Details
Ethyl methanesulfonate CAS 62-50-0View Details
62-50-0 -
 Ethyl methanesulfonate CAS 62-50-0View Details
Ethyl methanesulfonate CAS 62-50-0View Details
62-50-0 -
 99% Ethyl Methanesulfonate (working standard), Analytical GradeView Details
99% Ethyl Methanesulfonate (working standard), Analytical GradeView Details
62-50-0
