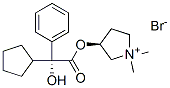
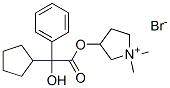
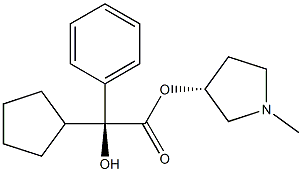
Glycopyrronium Bromide
- CAS NO.:51186-83-5
- Empirical Formula: C19H28NO3.Br
- Molecular Weight: 0
- MDL number: MFCD31381925
- EINECS: 209-887-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-04-22 09:42:11
What is Glycopyrronium Bromide?
Description
Glycopyrronium bromide or NVA 237 is a once-daily inhaled antimuscarinic bronchodilator newly approved by the European Commission for use in stable COPD. It is characterized by a quaternary ammonium structure, which reduces its oral bioavailability and, consequently, the systematic effects of any swallowed portion of the drug. Animal studies suggest that there are fewer cardiovascular effects compared to tiotropium because of the higher affinity to M3 versus M2 muscarinic receptors. Human studies showed fast onset of action, sustained bronchodilatation for 24 h, reduced dynamic hyperinflation of the lungs, and improved exercise tolerance. Glycopyrronium bromide in COPD airways clinical trial series (GLOW1–3) are the major clinical trials studying the efficacy and safety of glycopyrronium[1].
Chemical properties
White or almost white, crystalline powder.
The Uses of Glycopyrronium Bromide
erythro-Glycopyrronium Bromide is used in preparation of diester containing compounds for treating hyperhidrosis.
The Uses of Glycopyrronium Bromide
Anticholinergic
Definition
ChEBI: (2R,3S)-glycopyrronium bromide is a glycopyrronium bromide that has (2R,3S)-configuration. The racemate, ritropirronium bromide, is used for treatment of chronic obstructive pulmonary disease. It is an enantiomer of a (2S,3R)-glycopyrronium bromide.
Indications
Glycopyrronium bromide is indicated for use as a preoperative antimuscarinic to reduce salivary, tracheobronchial, and pharyngeal secretions; to reduce the volume and free acidity of gastric secretions, and to block cardiac vagal inhibitory reflexes during induction of anaesthesia and intubation when indicated. Glycopyrronium bromide may be used intraoperatively to counteract drug-induced or vagal traction reflexes with the associated arrhythmias. Glycopyrronium bromide protects against the peripheral muscarinic effects (e.g., bradycardia and excessive secretions) of cholinergic agents such as neostigmine and pyridostigmine given to reverse the neuromuscular blockade due to non-depolarising muscle relaxants.
Side Effects
The following reported adverse reactions are extensions of glycopyrronium bromide’s fundamentalpharmacological actions:
Cardiovascular: Tachycardia, ventricular fibrillation, bradycardia, palpitation and arrhythmia, hypertension, hypotension, cardiac arrest, heart block, prolonged QTc interval.
Dermatological: Flushing and inhibition of sweating. Severe allergic reactions or drug idiosyncrasies including urticaria and other dermal manifestations, pruritus, dry skin.
Gastrointestinal: Nausea, vomiting, dry mouth, constipation, taste alterations, including loss of taste.
Genitourinary: Urinary hesitancy and retention, impotence.
Ocular: Blurred vision due to mydriasis, cycloplegia, photophobia, increased ocular tension.
Nervous System: Inhibition of transmission at neuromuscular junction, headache, nervousness, drowsiness, dizziness, seizure, insomnia, some degree of mental confusion, especially in the elderly, hyperexcitability in children.
Pregnancy and perinatal: Suppression of lactation.
Respiratory System: Respiratory arrest.
General: Hyperpyrexia, bloated feeling, anaphylaxis/anaphylactoid reaction, hypersensitivity. Injection site reactions including pruritus, oedema, erythema, pain have been reported rarely.
References
[1] Alexander G. Mathioudakis, Victoria Chatzimavridou-Grigoriadou, Georgios A. Mathioudakis.“Chapter 16 - Drugs that act on the respiratory tract.” 2014: 241-256.
Properties of Glycopyrronium Bromide
| Melting point: | 175℃ |
| solubility | Freely soluble in water, soluble in ethanol (96 per cent), very slightly soluble in methylene chloride. |
| InChI | InChI=1/C19H28NO3.BrH/c1-20(2)13-12-17(14-20)23-18(21)19(22,16-10-6-7-11-16)15-8-4-3-5-9-15;/h3-5,8-9,16-17,22H,6-7,10-14H2,1-2H3;1H/q+1;/p-1/t17-,19-;/s3 |
Safety information for Glycopyrronium Bromide
Computed Descriptors for Glycopyrronium Bromide
| InChIKey | VPNYRYCIDCJBOM-SCHKGOGONA-M |
| SMILES | [N+]1(C)(C)CC[C@H](OC([C@](C2CCCC2)(O)C2=CC=CC=C2)=O)C1.[Br-] |&1:5,8,r| |
Glycopyrronium Bromide manufacturer
Equilife Laboratories Pvt Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 51186-83-5 Glycopyrronium Bromide / Glycopyrrolate 98%View Details
51186-83-5 Glycopyrronium Bromide / Glycopyrrolate 98%View Details
51186-83-5 -
 51186-83-5 98%View Details
51186-83-5 98%View Details
51186-83-5 -
![(3S)-rel-3-[[(2R)-2-cyclopentyl-2-hydroxy-2-phenylacetyl]oxy]-1,1-dimethyl-,Pyrrolidinium bromide (1:1) 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) (3S)-rel-3-[[(2R)-2-cyclopentyl-2-hydroxy-2-phenylacetyl]oxy]-1,1-dimethyl-,Pyrrolidinium bromide (1:1) 98%View Details
(3S)-rel-3-[[(2R)-2-cyclopentyl-2-hydroxy-2-phenylacetyl]oxy]-1,1-dimethyl-,Pyrrolidinium bromide (1:1) 98%View Details
51186-83-5 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0