eperezolid
- CAS NO.:165800-04-4
- Empirical Formula: C18H23FN4O5
- Molecular Weight: 394.4
- MDL number: MFCD00941980
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
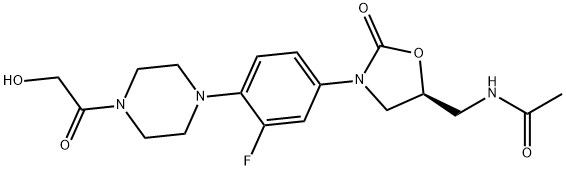
What is eperezolid?
The Uses of eperezolid
Eperezolid is an antimicrobial.
The Uses of eperezolid
Antibacterial.
Biological Activity
mic50: 0.5, 1.0, 2.0, 1.0, 16.0 and 2.0 mg/l for pcptostreptococcus, propionibacterium acnes, ciostridium pefringens, clostridium dijiicile, bactrroidesjagilis, and fusobacterium, respectivleyanaerobic bacteria are a common cause of serious infections. anaerobic species which predominate in clinical infections include the bacteroides fragilis group, clostridium spp. and peptostreptococcus spp. the oxazolidinones are a novel class of synthetic antimicrobials inhibiting the initiation of protein synthesis. two compounds of this class, eperezolid and linezolid have been shown to inhibit enterococcus faecalis and enterococcus faecium.
in vitro
ninety per cent of all tested propionibacterium acnes (30 strains), peptostreptococccuj spp. (50 strains), c. perjringens (50 strains) and c. dficile (50 strains) were inhibited by <2 mg/l eperezolid. linezolid showed higher activity (mic90 4.0 mg/l) against b. jragilis (100 strains) compared to eperezolid (mic90 16 mg/l) [1].
in vivo
the in vivo effectiveness of eperezolid and linezolid against one strain each of enterococcus faecalis and vancomycin-resistant enterococcus faecium was examined in a rat intraabdominal abscess model. eperezolid was ineffective at doses of 25 mg/kg of body weight twice daily for the reductions in abscess bacterial density for e. faecalis. against e. faecium infections, intravenous eperezolid was effective, reducing densities approximately 2 log10 cfu/g [2].
References
[1] edlund c, oh h, nord ce. in vitro activity of linezolid and eperezolid against anaerobic bacteria. clin microbiol infect. 1999;5(1):51-53.
[2] schülin t, thauvin-eliopoulos c, moellering rc jr, eliopoulos gm. activities of the oxazolidinones linezolid and eperezolid in experimental intra-abdominal abscess due to enterococcus faecalis or vancomycin-resistant enterococcus faecium. antimicrob agents chemother. 1999;43(12):2873-6.
[3] schaadt rd, batts dh, daley-yates pt, pawsey sd, stalker dj, zurenko ge. serum inhibitory titers and serum bactericidal titers for human subjects receiving multiple doses of the antibacterial oxazolidinones eperezolid and linezolid. diagn microbiol infect dis. 1997;28(4):201-4.
Properties of eperezolid
| Melting point: | 174-176℃ |
| Boiling point: | 701.2±60.0 °C(Predicted) |
| Density | 1.370±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | insoluble in EtOH; insoluble in H2O; ≥14.05 mg/mL in DMSO |
| form | Powder |
| pka | 13.84±0.10(Predicted) |
| color | White to off-white |
Safety information for eperezolid
Computed Descriptors for eperezolid
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![Ethyl 1-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]cyclopropane-1-carboxylate](https://img.chemicalbook.in/CAS/GIF/1257213-52-7.gif)


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8