AZD6738
- CAS NO.:1352226-88-0
- Empirical Formula: C20H24N6O2S
- Molecular Weight: 412.51
- MDL number: MFCD28952790
- EINECS: 805-672-6
- Update Date: 2024-11-17 16:00:36
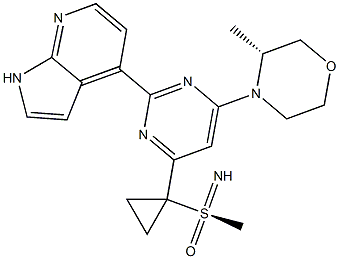
What is AZD6738?
Description
AZD 6738 is an orally bioavailable inhibitor of the serine/threonine protein kinase ataxia telangiectasia mutated (ATM) and Rad3-related (ATR; IC50 = 1 nM). It can inhibit ATR substrate Chk1 Ser345 phosphorylation in cells with an IC50 value of 74 nM. AZD 6738 has been shown to inhibit the proliferation of various solid and hematological cell lines with IC50 values of less than 1 μM and to reduce tumor growth in several ATM-deficient xenograft models. When used in combination with either DNA damaging chemotherapy agents or ionizing radiation, AZD 6738 demonstrated synergistic cell killing activity across multiple cell lines in vitro and in xenograft studies.
The Uses of AZD6738
AZD6738 is an orally available morpholino-pyrimidine-based inhibitor of ataxia telangiectasia and rad3 related (ATR) kinase, with potential antineoplastic activity. Upon oral administration, ATR kinase inhibitor AZD6738 selectively inhibits ATR activity by blocking the downstream phosphorylation of the serine/threonine protein kinase CHK1. This prevents ATR-mediated signaling, and results in the inhibition of DNA damage checkpoint activation, disruption of DNA damage repair, and the induction of tumor cell apoptosis. In addition, AZD6738 sensitizes tumor cells to chemo- and radiotherapy. ATR, a serine/threonine protein kinase upregulated in a variety of cancer cell types, plays a key role in DNA repair, cell cycle progression, and survival; it is activated by DNA damage caused during DNA r
What are the applications of Application
Ceralasertib (AZD6738) is a highly selective and potent ATR inhibitor that serves as a key sensor for the detection of DNA damage and a key activator of DNA damage checkpoints. Ceralasertib is currently in clinical development studies for the treatment of metastatic desmoplasia-resistant prostate cancer (mCRPC), advanced/metastatic melanoma, non-small cell lung cancer (NSCLC), prolymphocytic leukemia (PLL), and hairy cell leukemia (HCL), among other solid tumours.
Mechanism of action
The anti-tumor effects of an ATR inhibitor, AZD6738, have been investigated in vitro using human gastric cancer cell lines and an in vivo xenograft model. AZD6738 was found to inhibit the proliferation of gastric cancer cells with dysfunctional ATMs by suppressing proliferative signaling and blocking cell-cycle progression in the S phase. Furthermore, AZD6738 disrupted HR-mediated DNA repair in sensitive cells, whereas ATR inhibition activated the ATM-Chk2 pathway to promote the repair of DNA damage induced by AZD6738 in insensitive cells with functional ATM. ATM activation is the main resistance mechanism to AZD6738, and ATM and ATR act compensatively. The study also demonstrates that AZD6738 attenuates ATR activity and induces ATM activation, thus promoting an ATR to ATM switch in the presence of DNA damage. These findings show the interaction between AZD6738 has a synthetic lethal interaction with ATM defect in gastric cancer cells and that ATM inactivation in ATM dysfunctional SNU-601 cells is due to HDAC1 deficiency[4].
Side Effects
Common side effects of AZD6738 include: anaemia, thrombocytopenia, neutropenia , increased risk of infection, bruising and bleeding, dizziness diarrhoea, loss of appetite, changes in kidney function, tiredness Feeling unwell or sickChanges in kidney function, feeling weak and lack of energy.
Enzyme inhibitor
This orally active, and selective ATR kinase inhibitor (FW = 412.51 g/mol; CAS 1352226-88-0; Solubility: 80 mg/mL DMSO; < 1 mg/mL H2O) targets Ataxia Telangiectasia/Rad3-related kinase (IC50 = 1 nM), a serine/threonine protein kinase that activates checkpoint signaling upon genotoxic stresses (e.g., ionizing radiation, ultraviolet light, or replication stalling), thereby serving as a DNA damage sensor. In a model consisting of primary human Chronic Myelogenous Leukemia (CL) cells with biallelic TP53 or ATM inactivation xenotransplanted into NOD/Shi scid/IL-2Rγ mice, AZD6738 provides potent and specific inhibition of ATR signalling with compensatory activation of ATM/p53 pathway in cycling CLL cells in the presence of genotoxic stress. In p53 or ATM defective cells, AZD6738 treatment results in replication fork stalls and accumulation of unrepaired DNA damage, as evidenced by γH2AX and 53BP1 foci formation, carried through into mitosis and resulting in cell death by mitotic catastrophe. AZD6738 displays selective cytotoxicity towards ATM- or p53-deficient CLL cells. AZD6738 also potentiates cisplatin and gemcitabine cytotoxicity in Non-Small-Cell Lung Carcinoma (NSCLC) cell lines with intact ATM kinase signaling. It also potently synergizes with cisplatin in ATM-deficient NSCLC cells. When used in combination, cisplatin and AZD6738 resolve ATM-deficient lung cancer xenografts.
References
[1]. choi my, fecteau jf, brown j, et al. induction of proliferation sensitizes chronic lymphocytic leukemia cells to apoptosis mediated by the atr inhibitor azd6738. cancer research, 2014, 74(19 supplement): 5485-5485.
[2]. checkley s, maccallum l, yates j, et al. bridging the gap between in vitro and in vivo: dose and schedule predictions for the atr inhibitor azd6738. scientific reports, 2015, 5.
[3]. guichard sm, brown e, odedra r, et al. the pre-clinical in vitro and in vivo activity of azd6738: a potent and selective inhibitor of atr kinase. cancer research, 2013, 73(8 supplement): 3343-3343.
[4] A. Min. “AZD6738, A Novel Oral Inhibitor of ATR, Induces Synthetic Lethality with ATM Deficiency in Gastric Cancer Cells.” Molecular Cancer Therapeutics 16 1 (2017): 566–577.
Properties of AZD6738
| Density | 1.52±0.1 g/cm3(Predicted) |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 12.53±0.40(Predicted) |
| color | Pale Yellow |
Safety information for AZD6738
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for AZD6738
| InChIKey | OHUHVTCQTUDPIJ-YEMGFFRQNA-N |
| SMILES | C12NC=CC1=C(C1=NC(N3CCOC[C@H]3C)=CC(C3([S@](C)(=N)=O)CC3)=N1)C=CN=2 |&1:14,19,r| |
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![2-(2-Chlorophenyl)-4-(3-(diMethylaMino)phenyl)-5-Methyl-1H-pyrazolo[4,3-c]pyridine-3,6(2H,5H)-dione](https://img.chemicalbook.in/CAS/20150408/GIF/1218942-37-0.gif)
![4-BroMo-N-(4-broMophenyl)-3-[[(phenylMethyl)aMino]sulfonyl]benzaMide](https://img.chemicalbook.in/CAS/GIF/312756-74-4.gif)
You may like
-
 873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1